PEM Suite is the result of a systematic co-creation methodology involving over 150+ people (patients, healthcare professionals, patient representatives, life sciences representatives, regulators) working together to build a framework for patient engagement now widely accepted, used and endorsed.
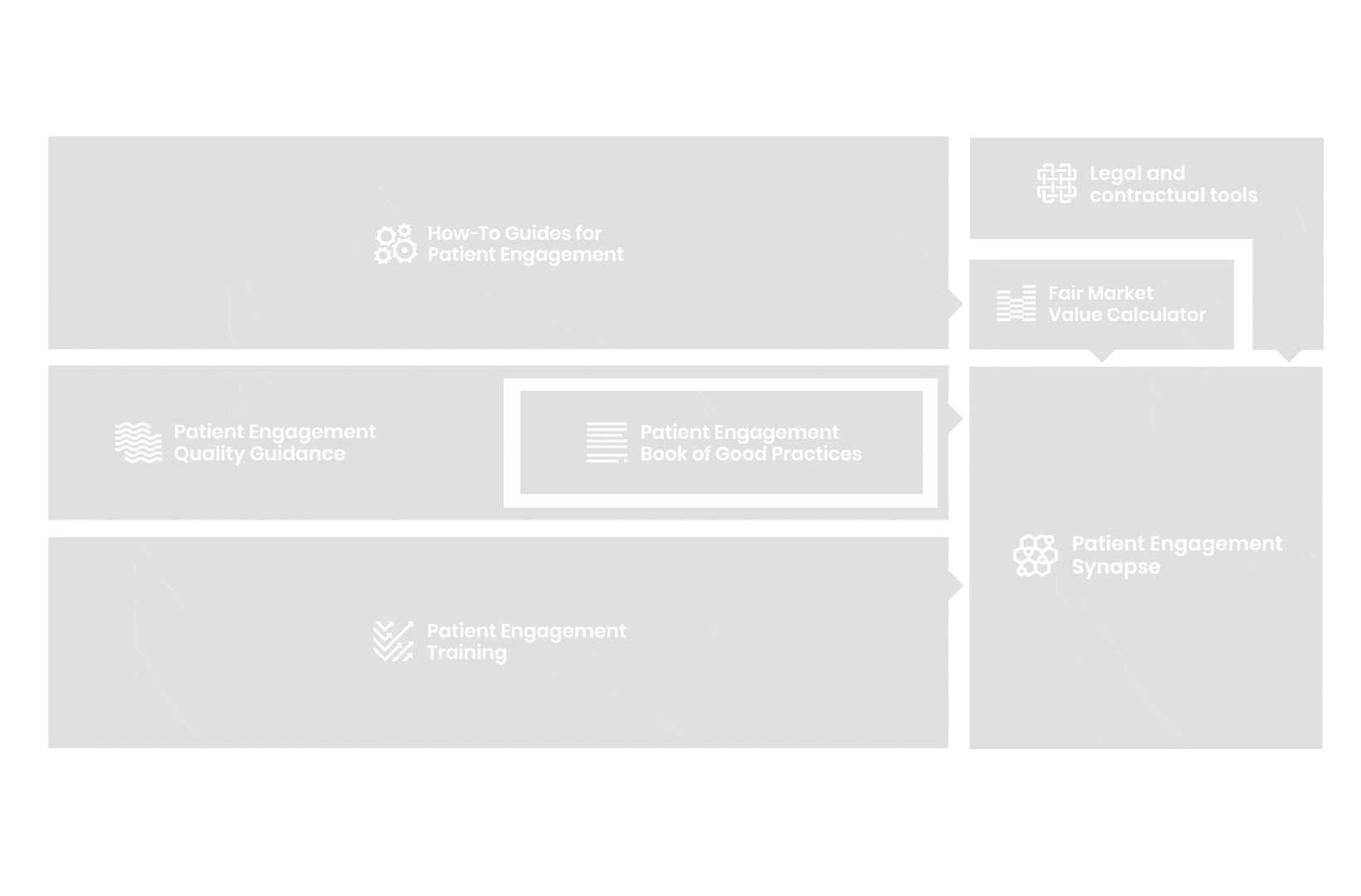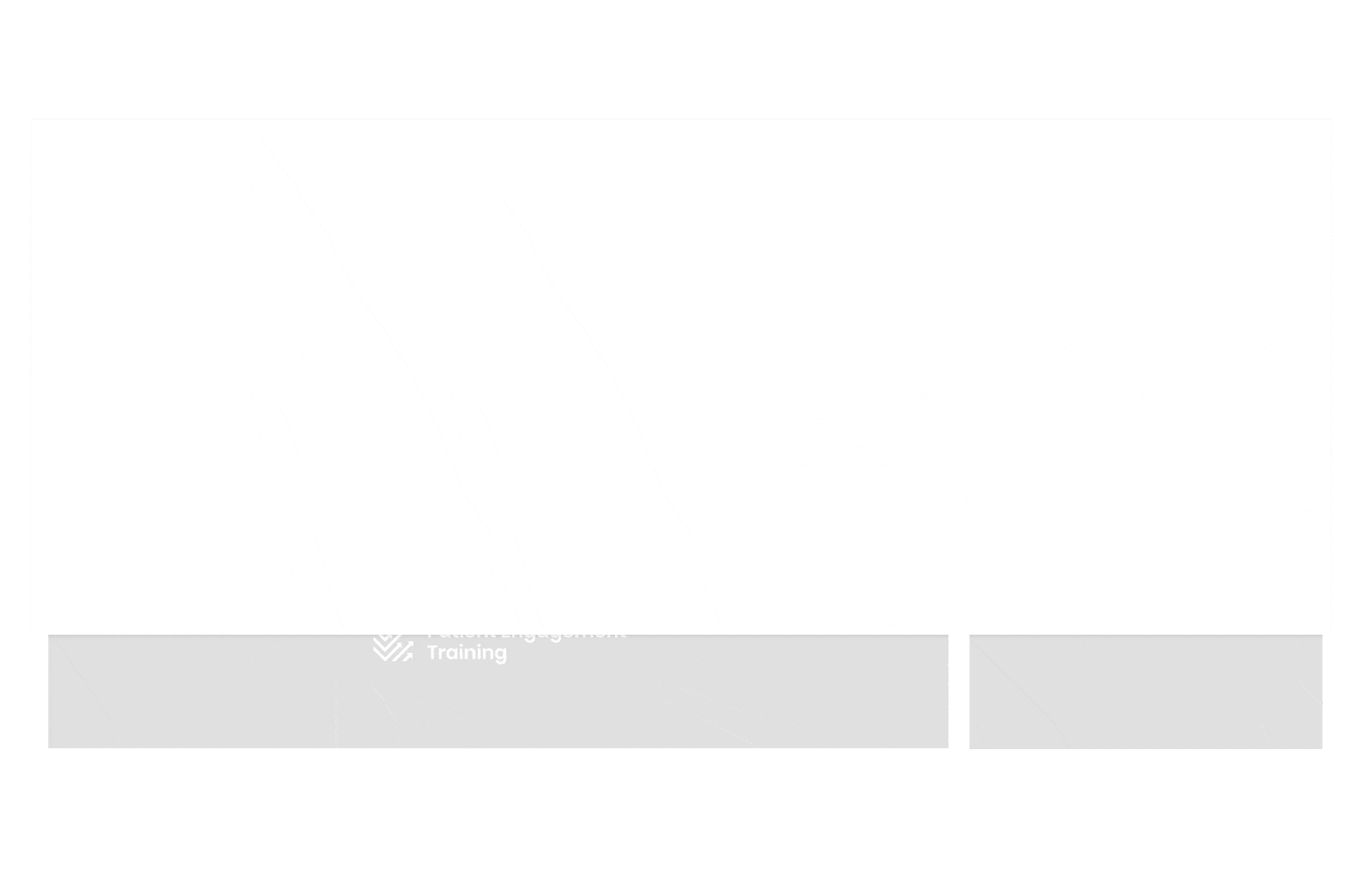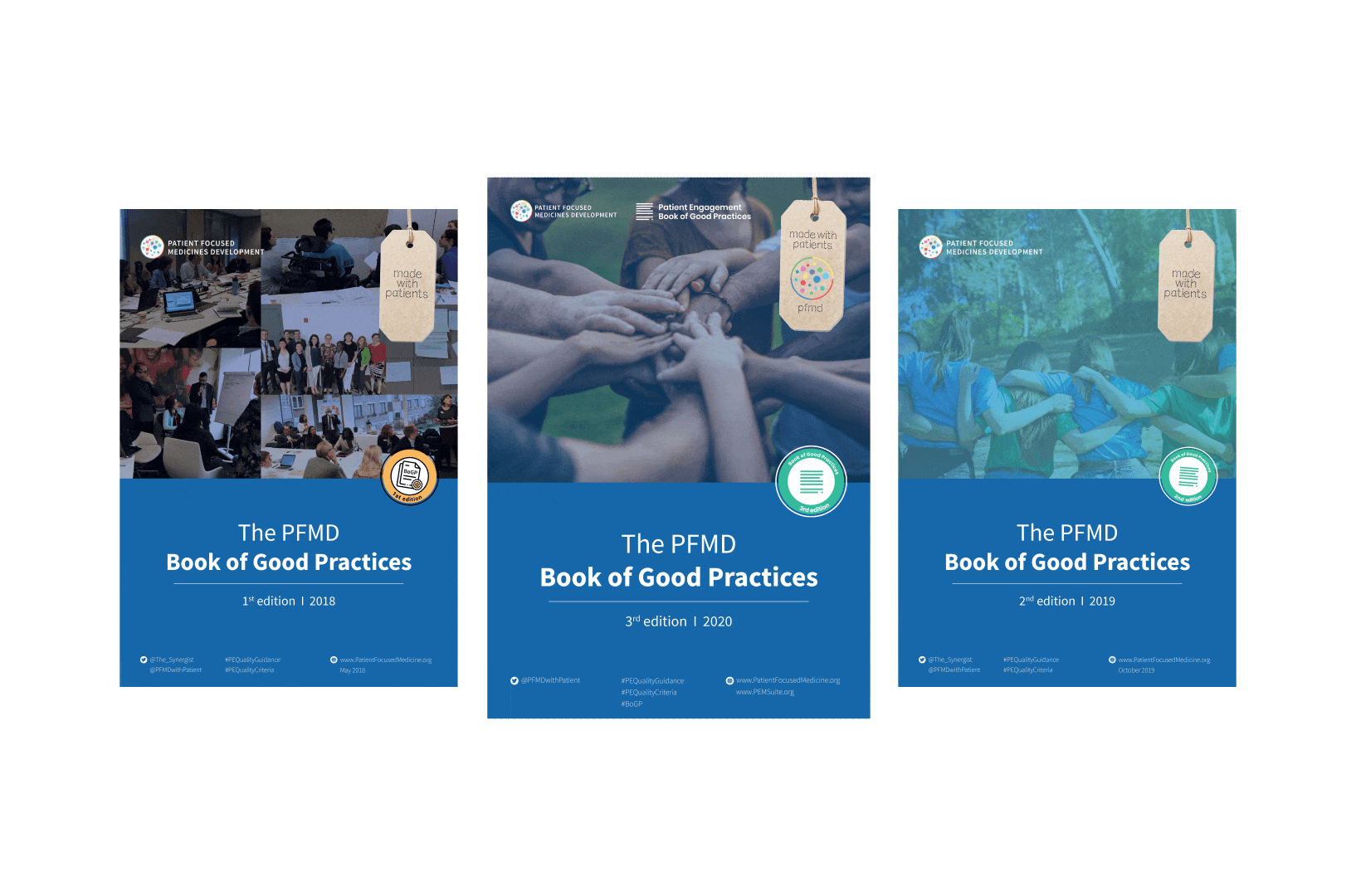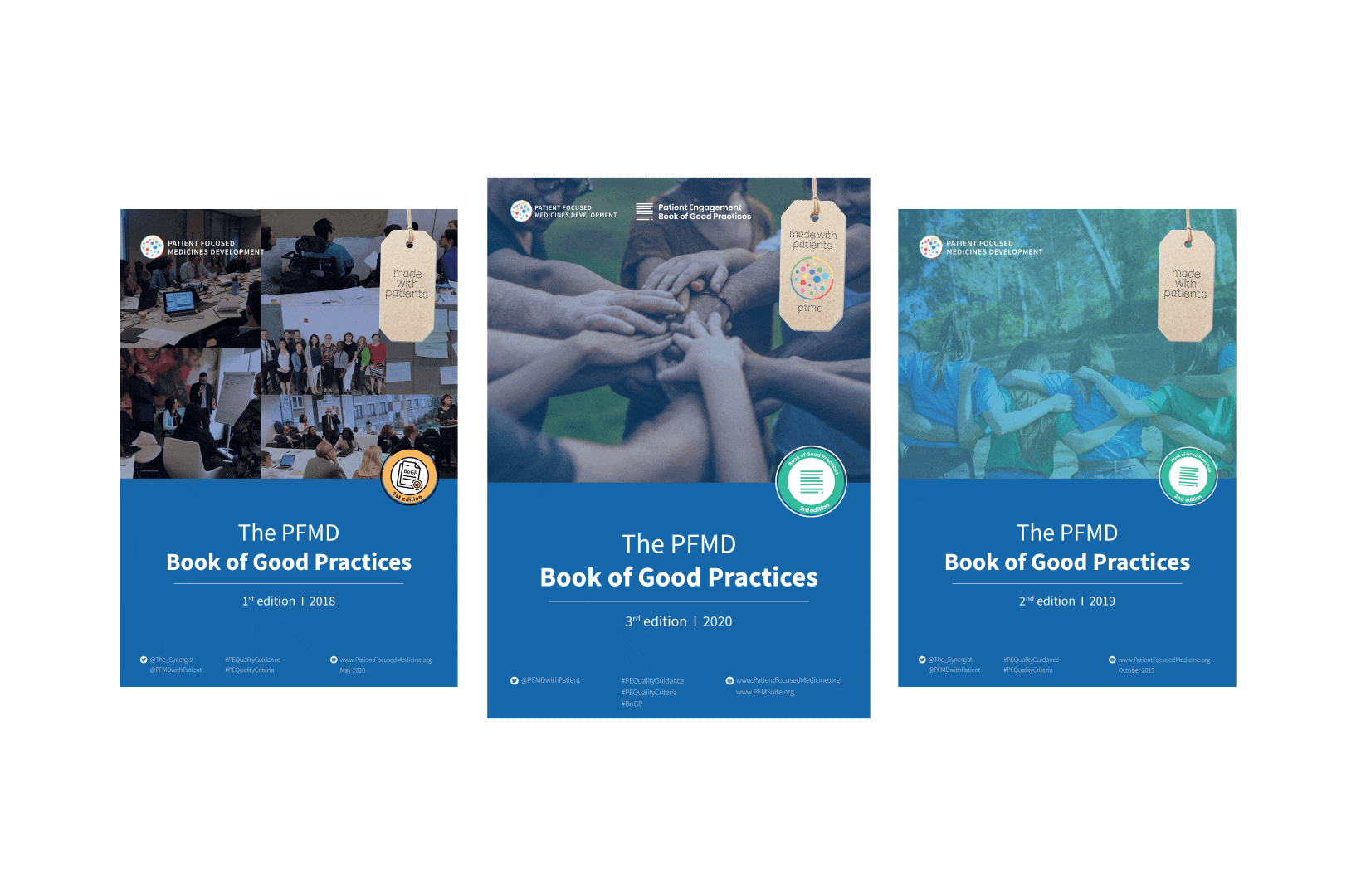
A hub of co-created tools, resources and practices to help stakeholders adopt patient engagement in a systematic, efficient and meaningful way.

By delivering faster new therapies that better address unmet needs and working together throughout the lifecycle of a product, patient engagement leads to better health outcomes and better-performing health stakeholders.

However, a fragmented landscape and lack of comprehensive guidance are disrupting community efforts to make patient engagement the norm.
You can read more about the need for systematic patient engagement here.

That’s why, since 2016, key stakeholders have worked together, as part of the Patient Focused Medicines Development (PFMD) initiative,
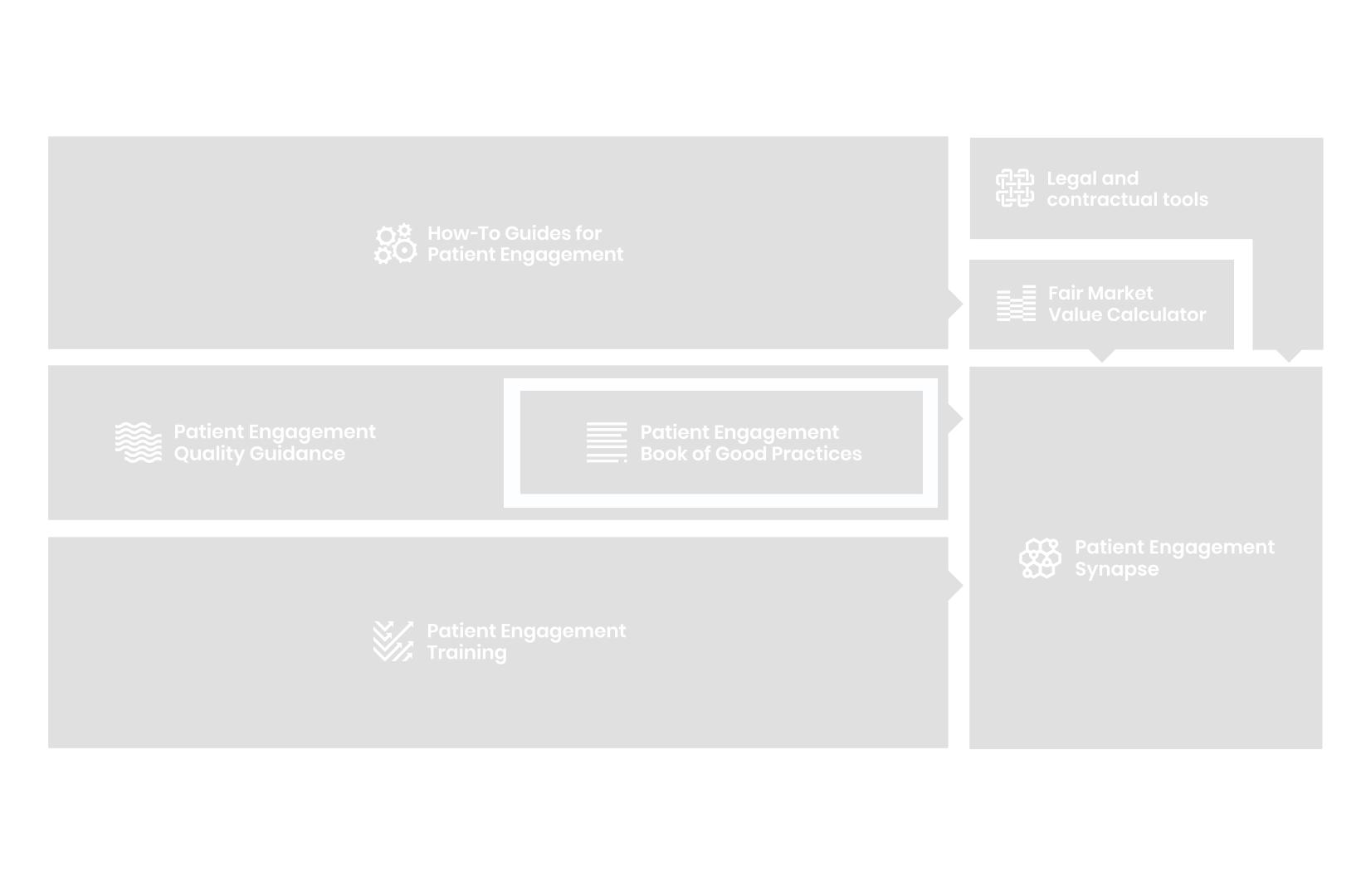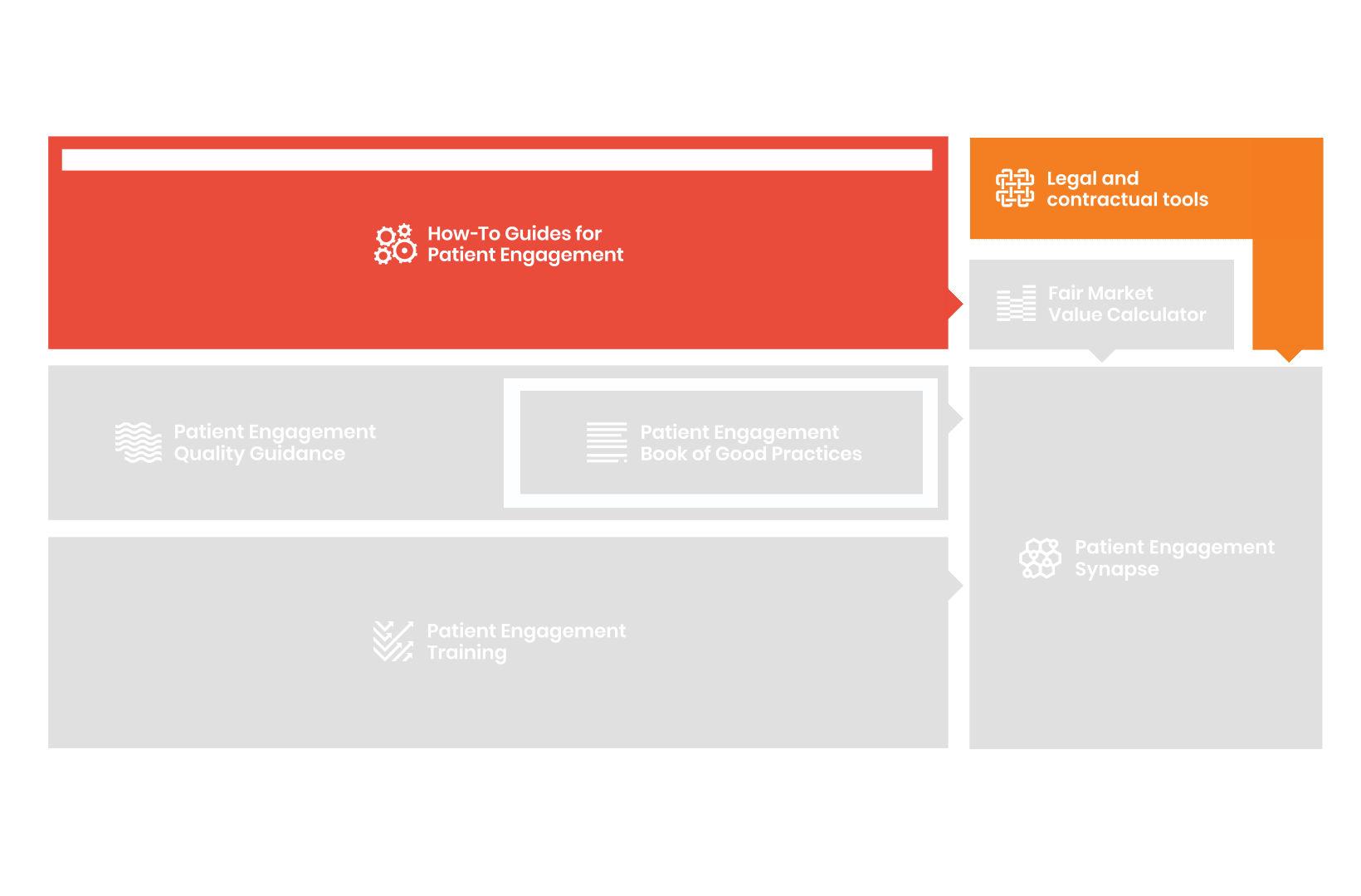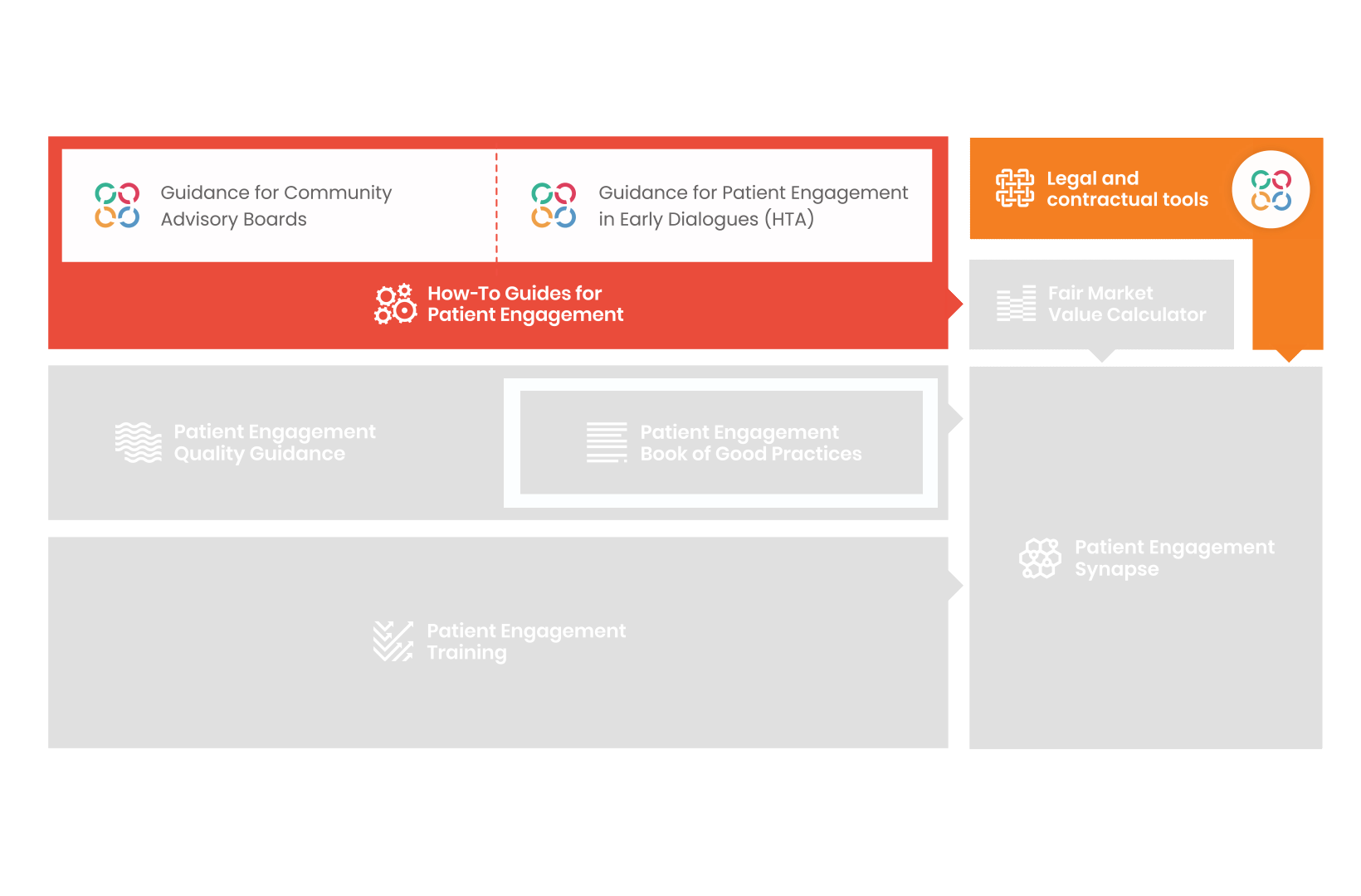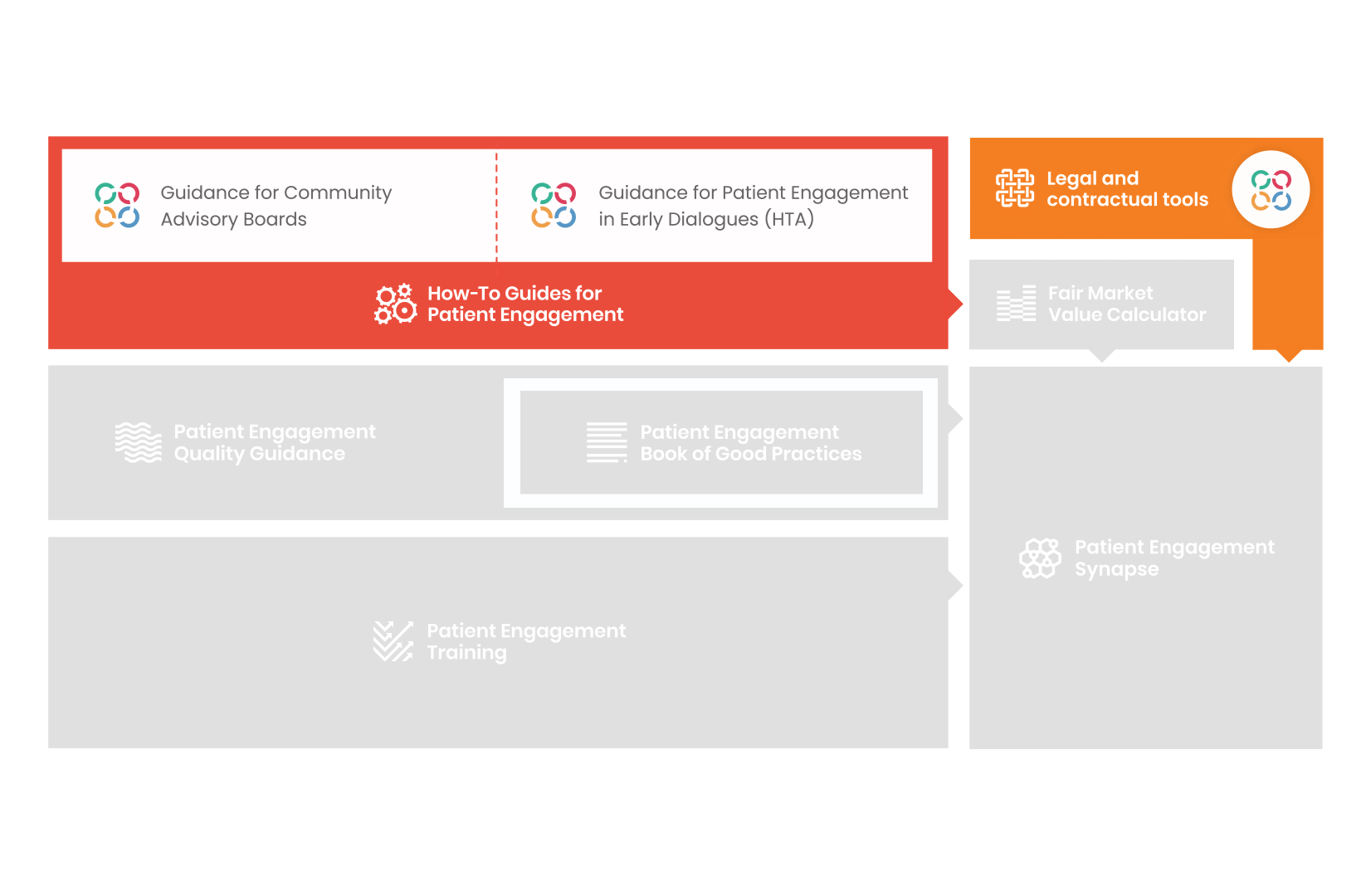
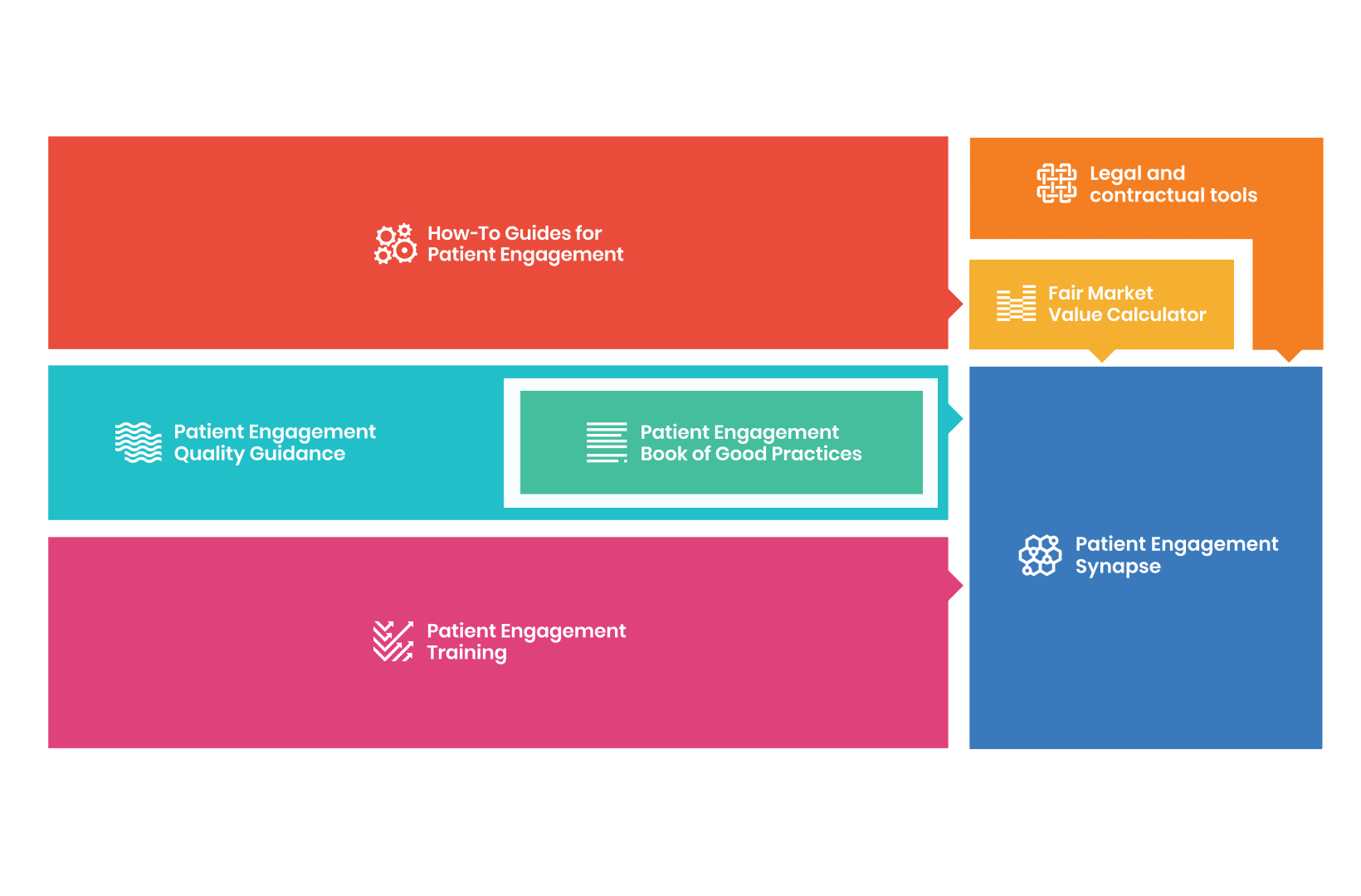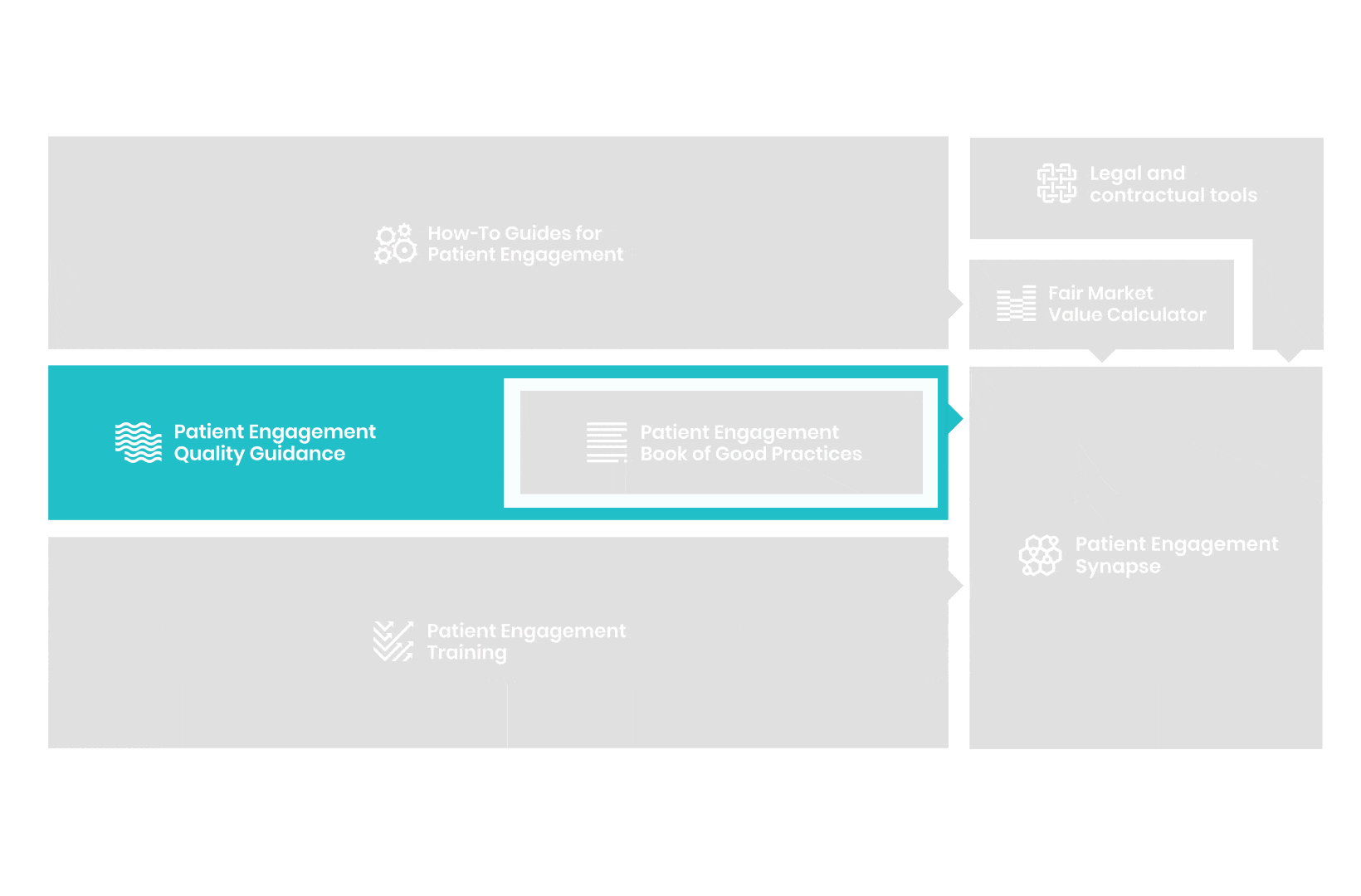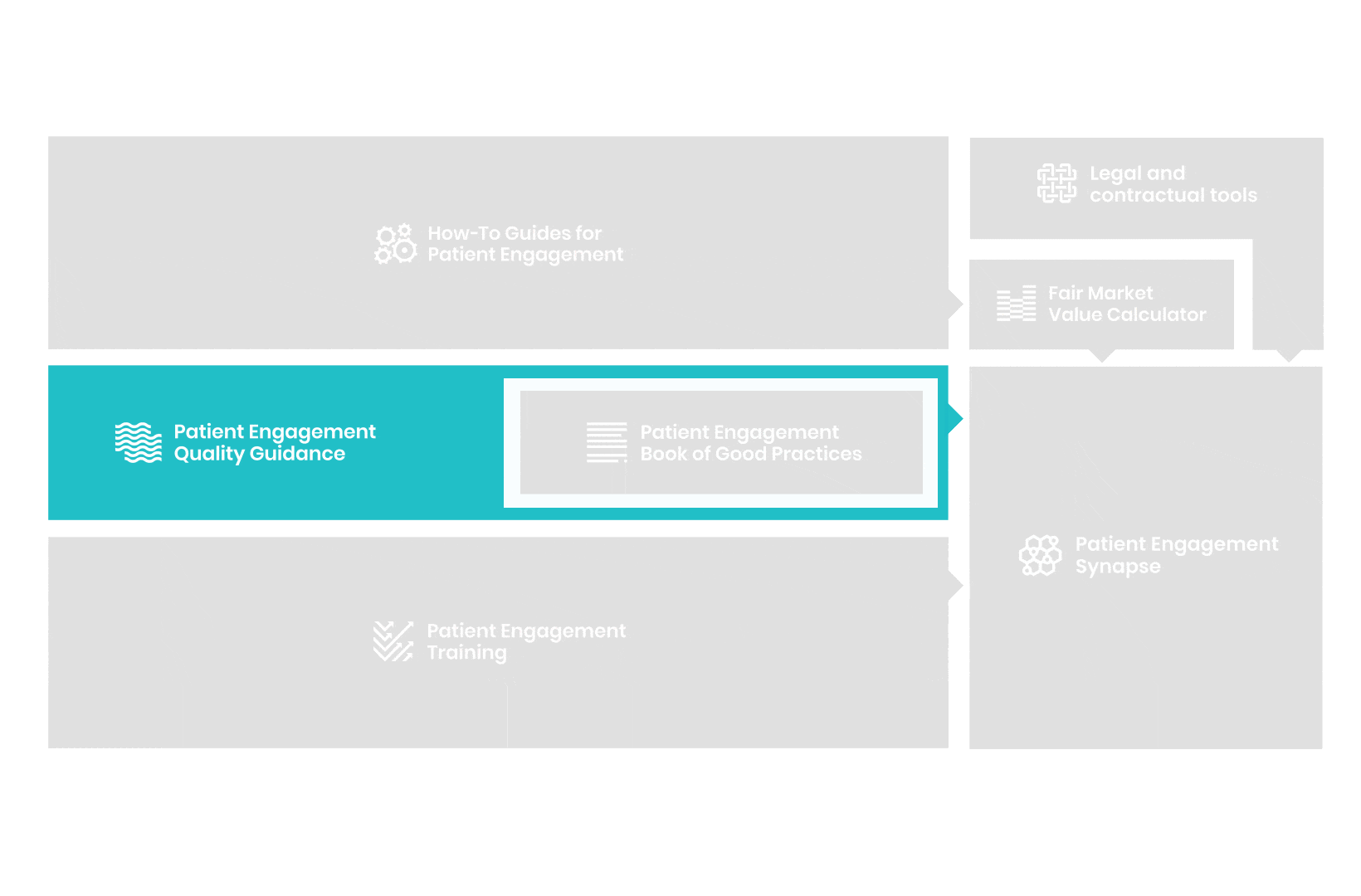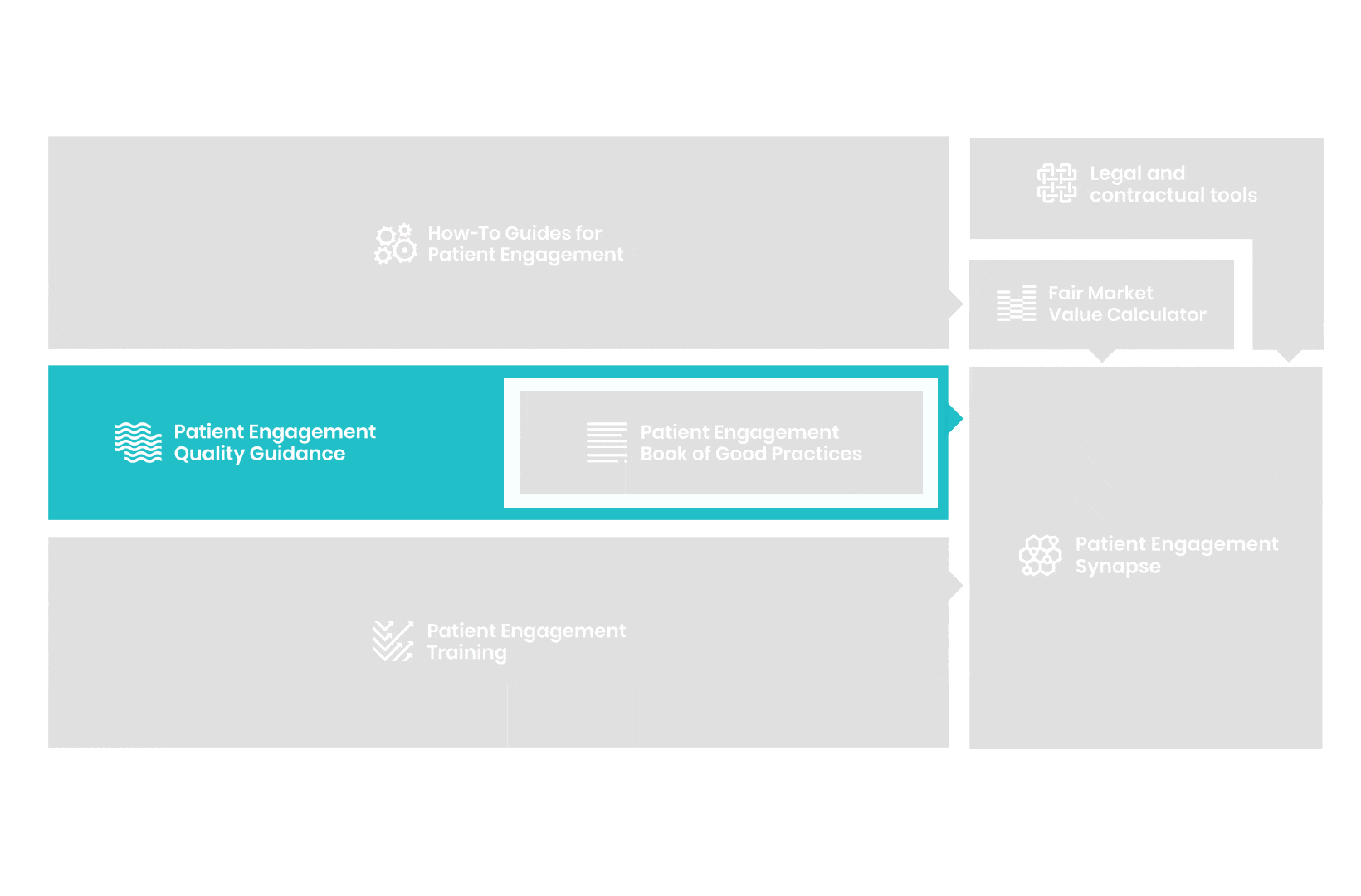
to explore the patient engagement landscape and build a patient engagement master framework and its toolbox, the Patient Engagement Management Suite – a set of integrated tools to help to make patient engagement happen.

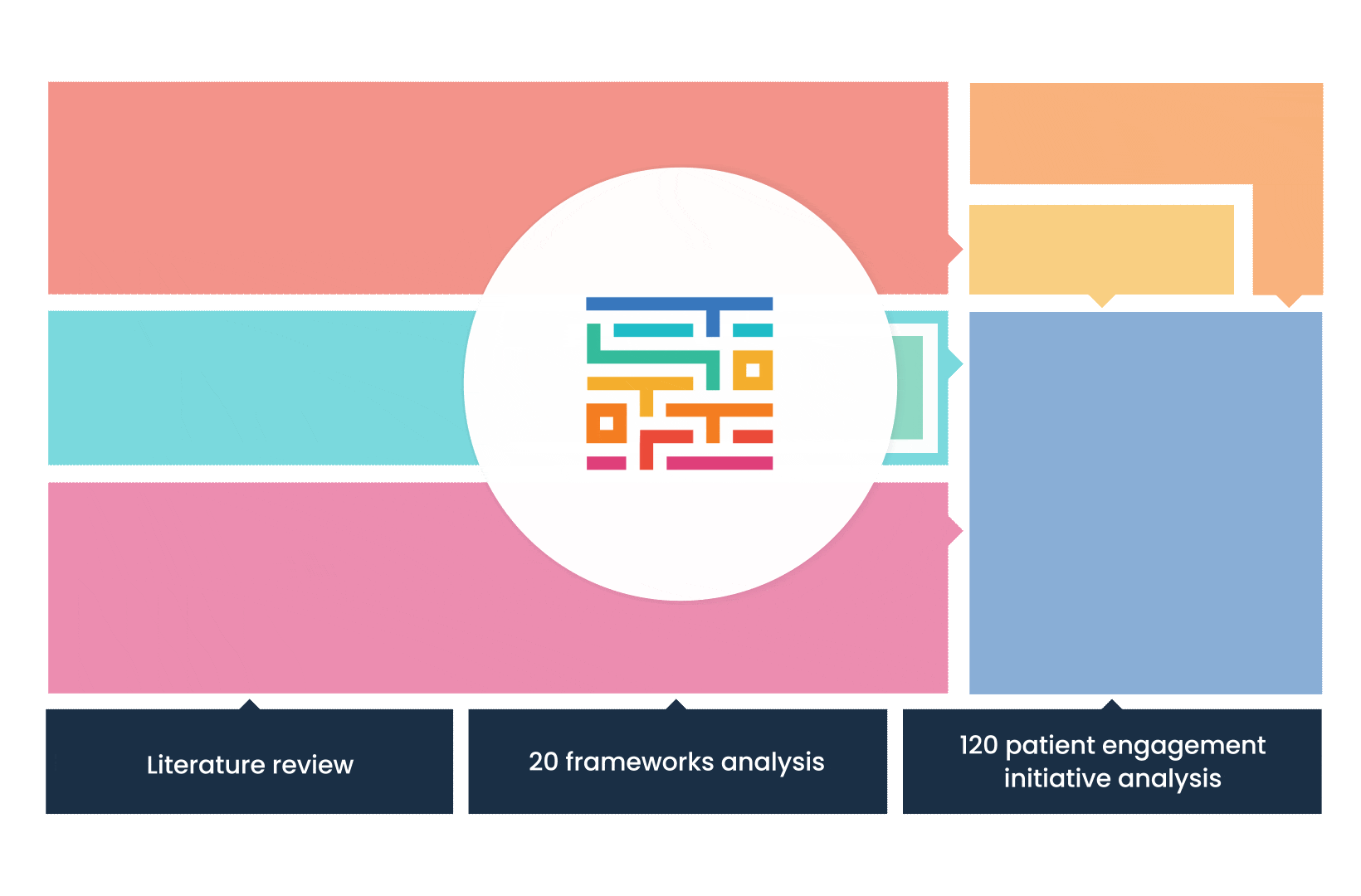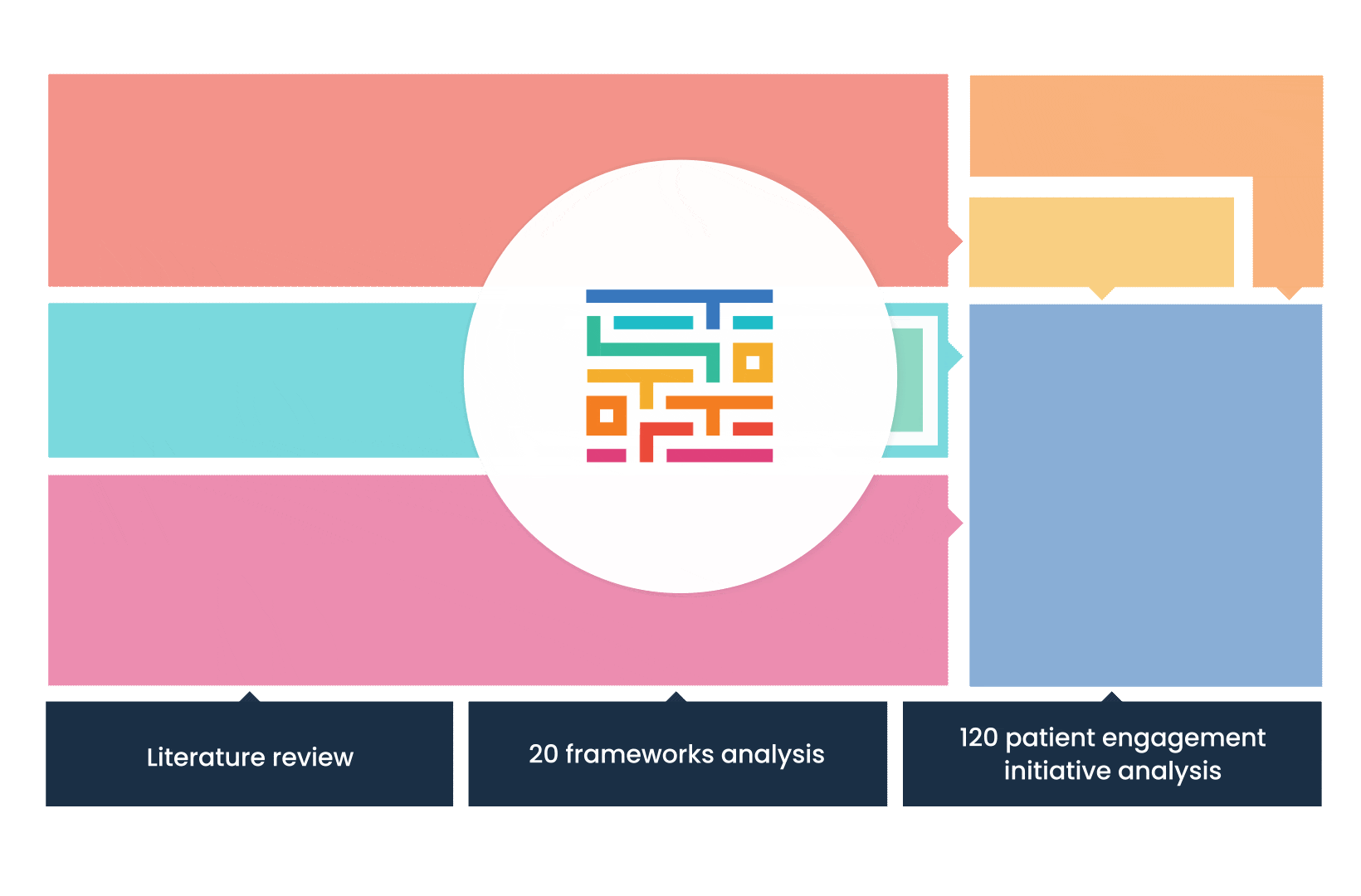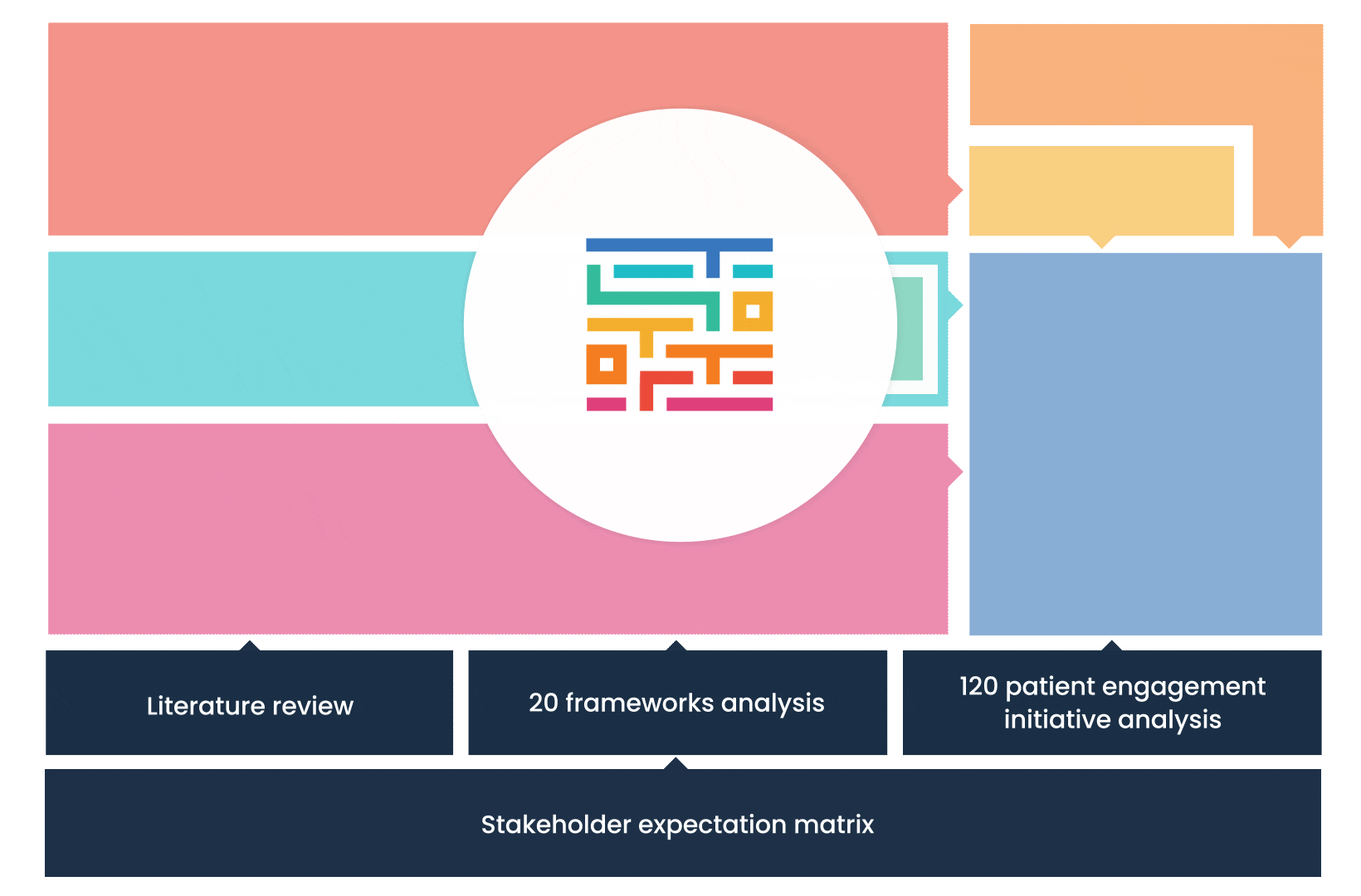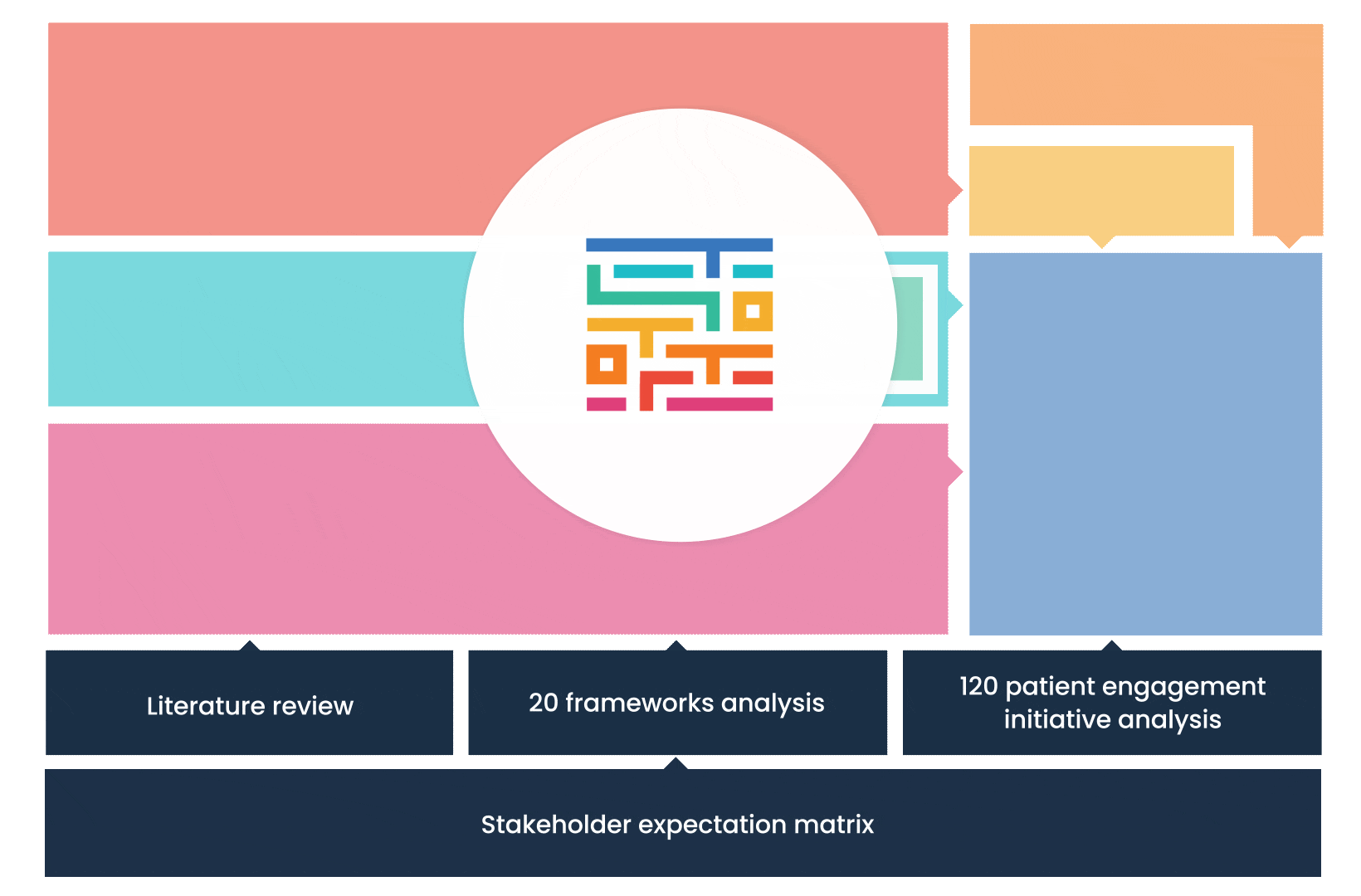
The process started with an extensive literature review, analysis of 20 existing frameworks and mapping of 120 initiatives demonstrating practical patient engagement.
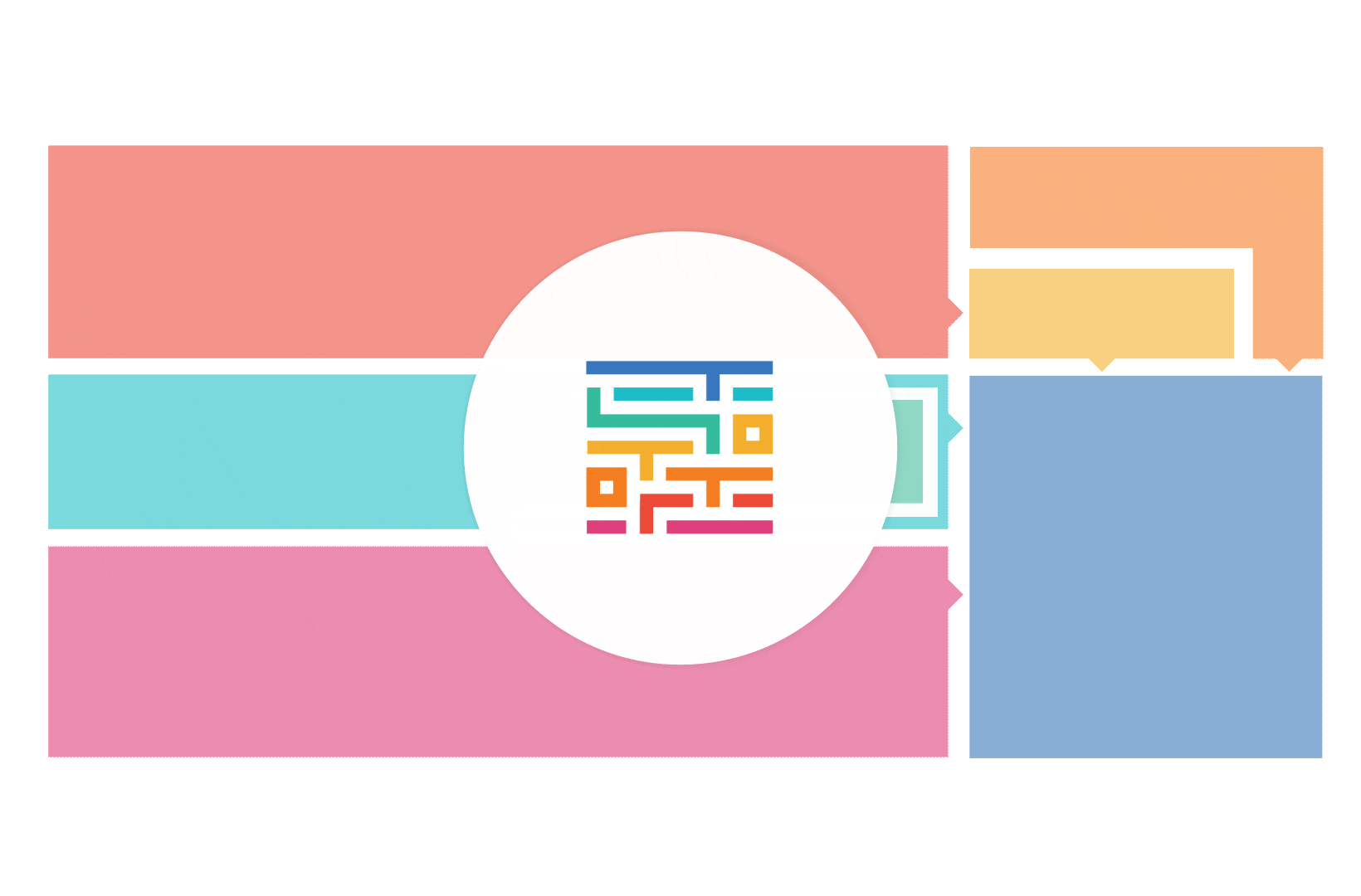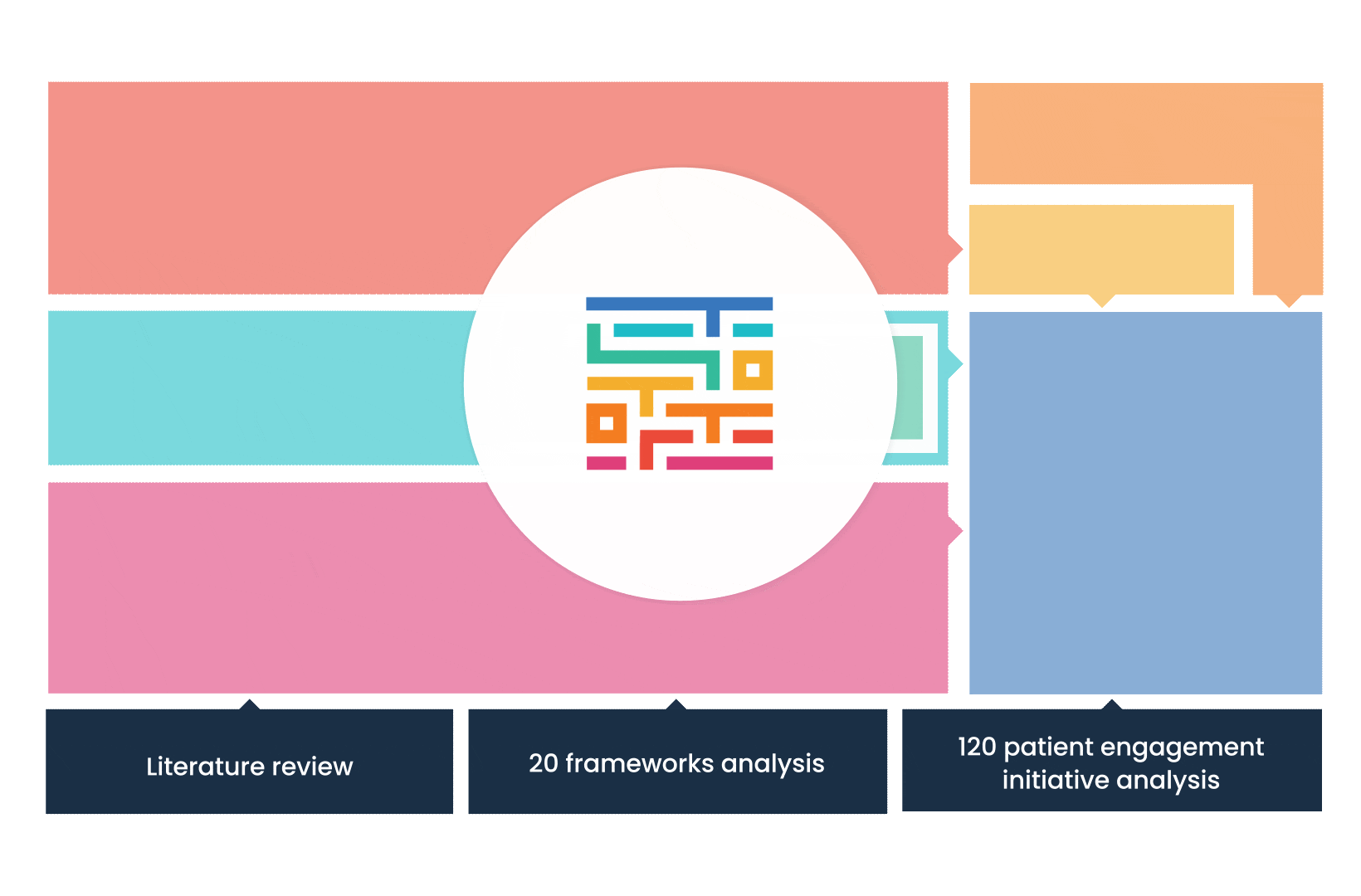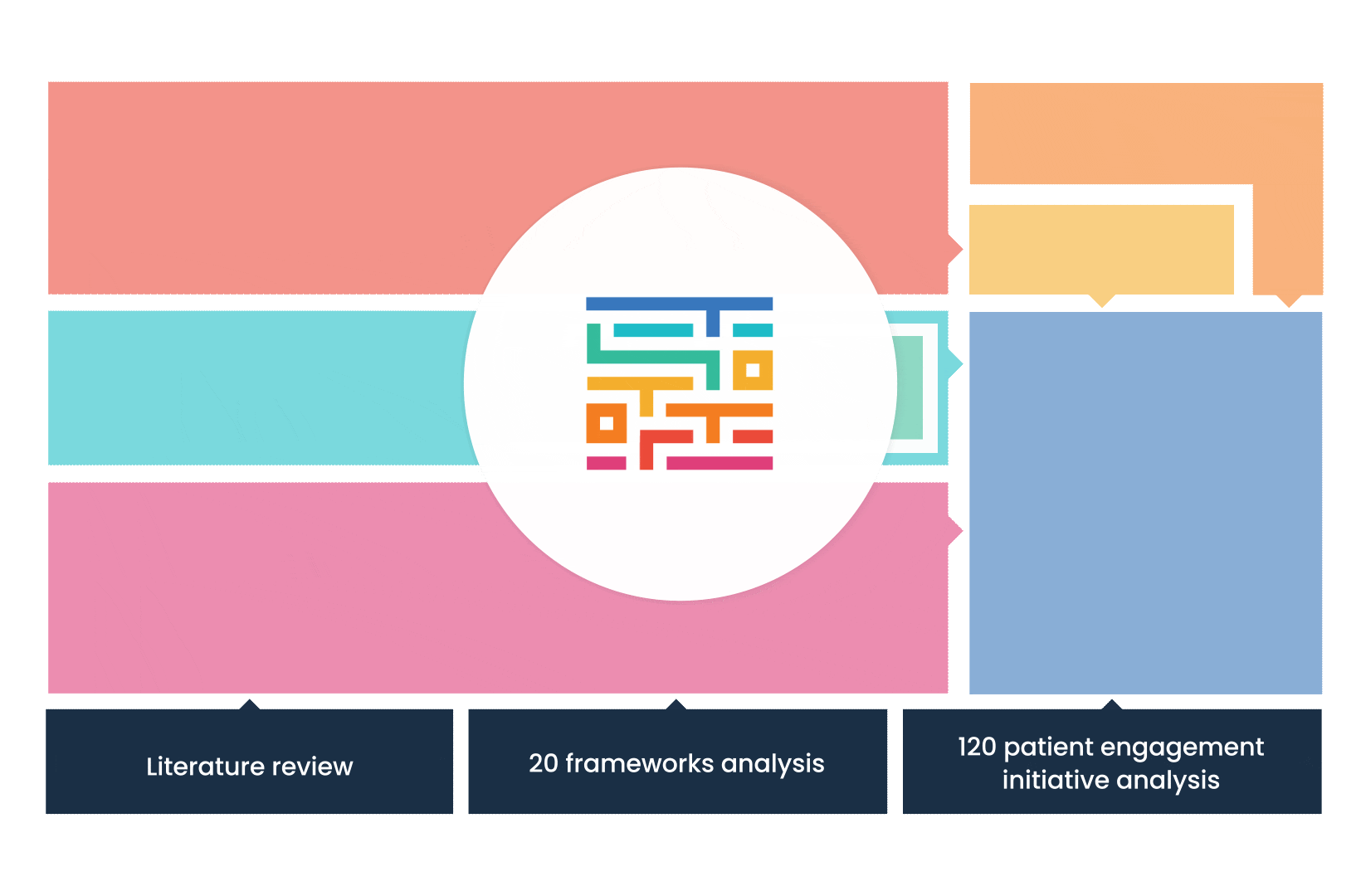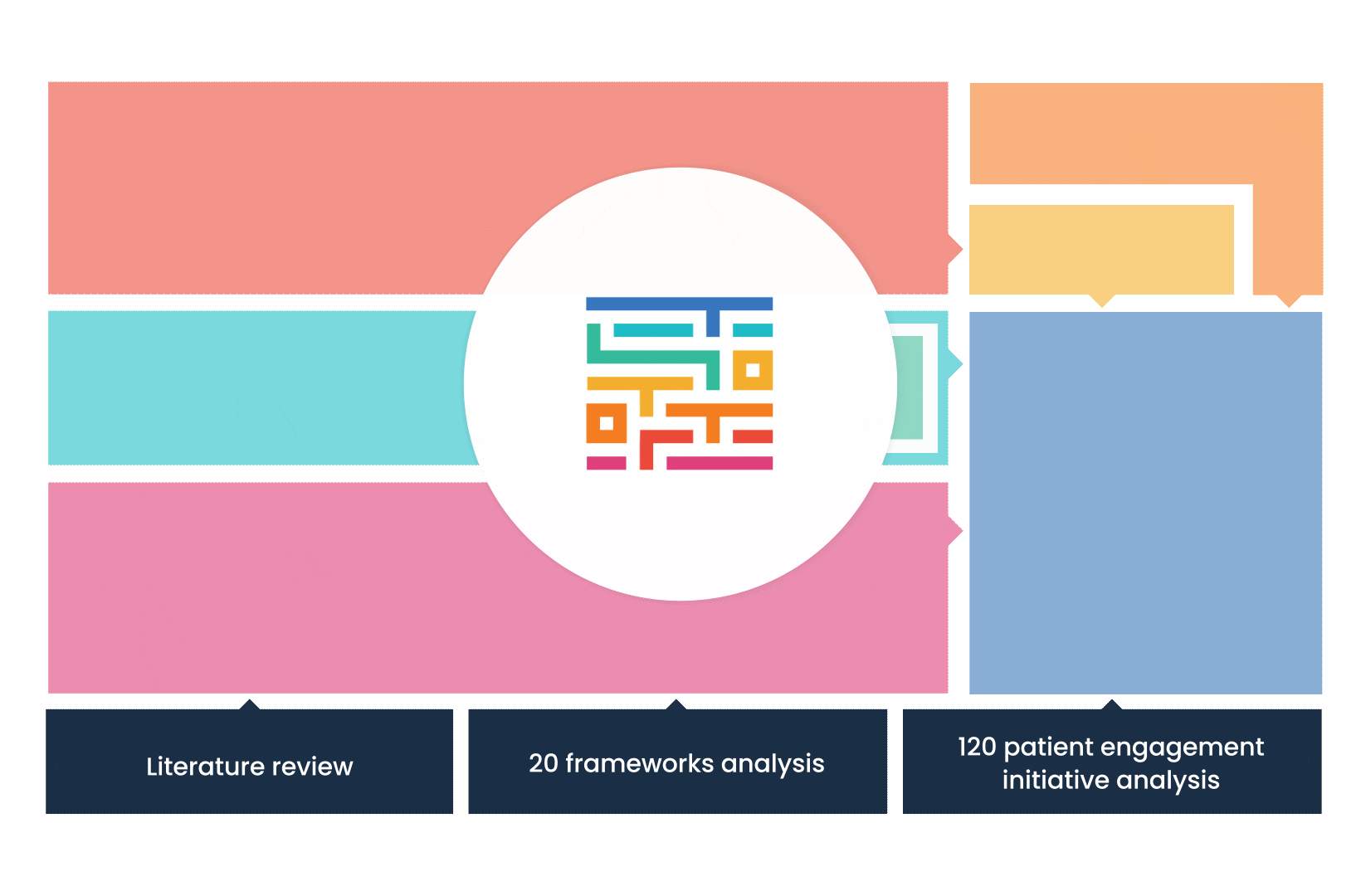
Next, a series of interviews were conducted, generating a stakeholders’ expectations matrix that identified the needs and preferences of different stakeholders for patient engagement.
The full publication is available online. It was concluded that, while there is an appetite for patient involvement in drug development, there is no detailed guidance on how to make patient engagement happen.
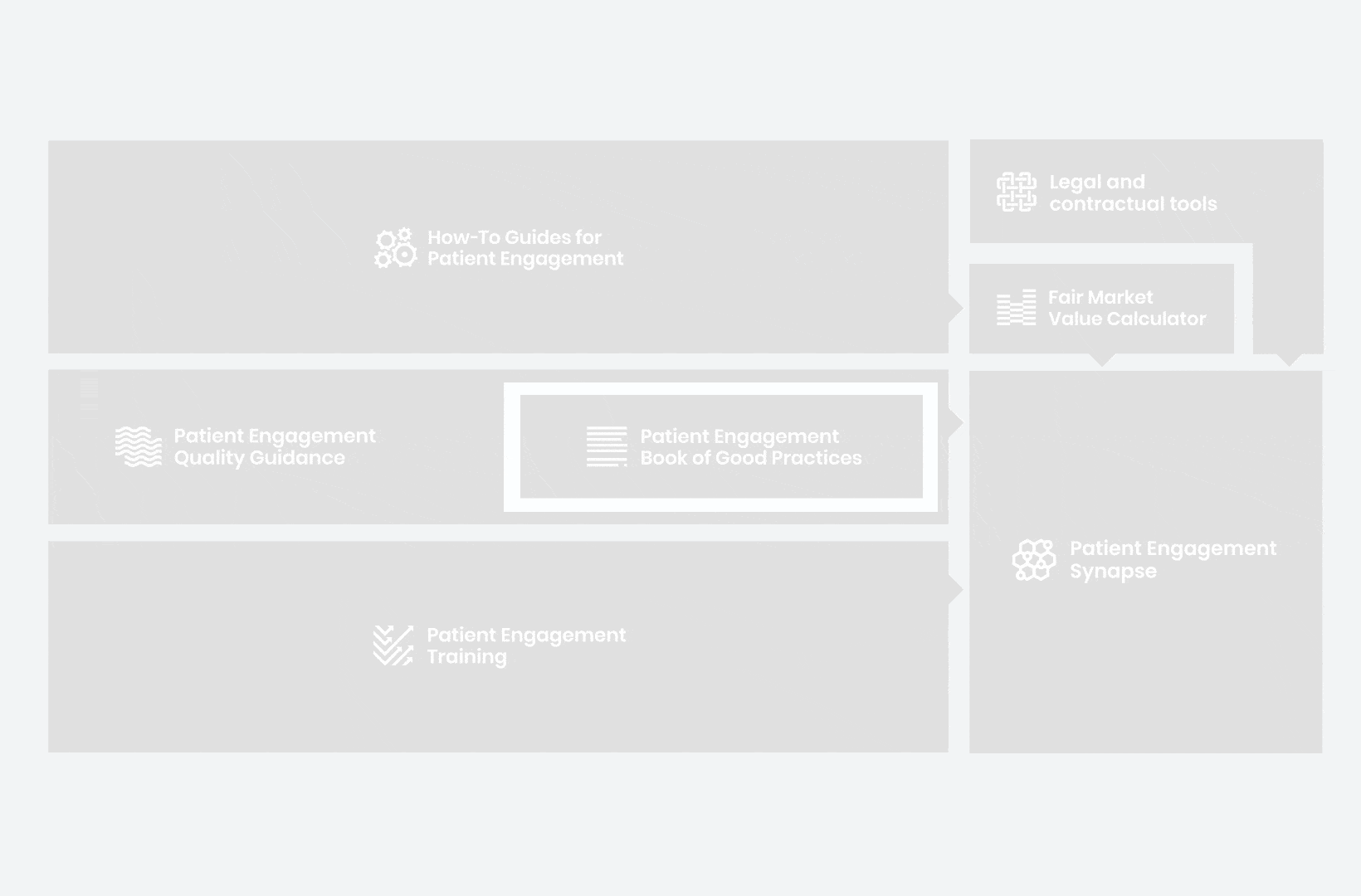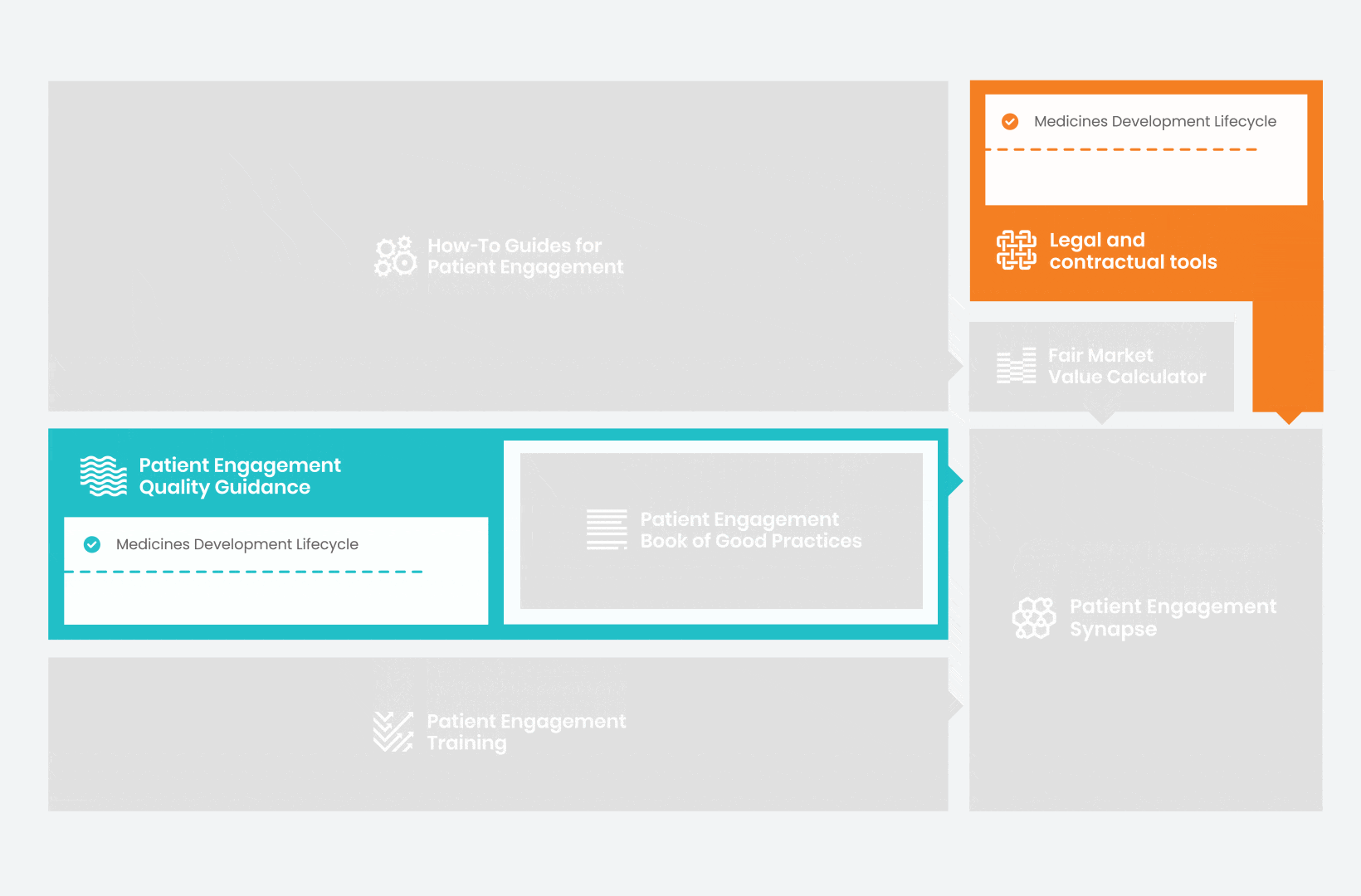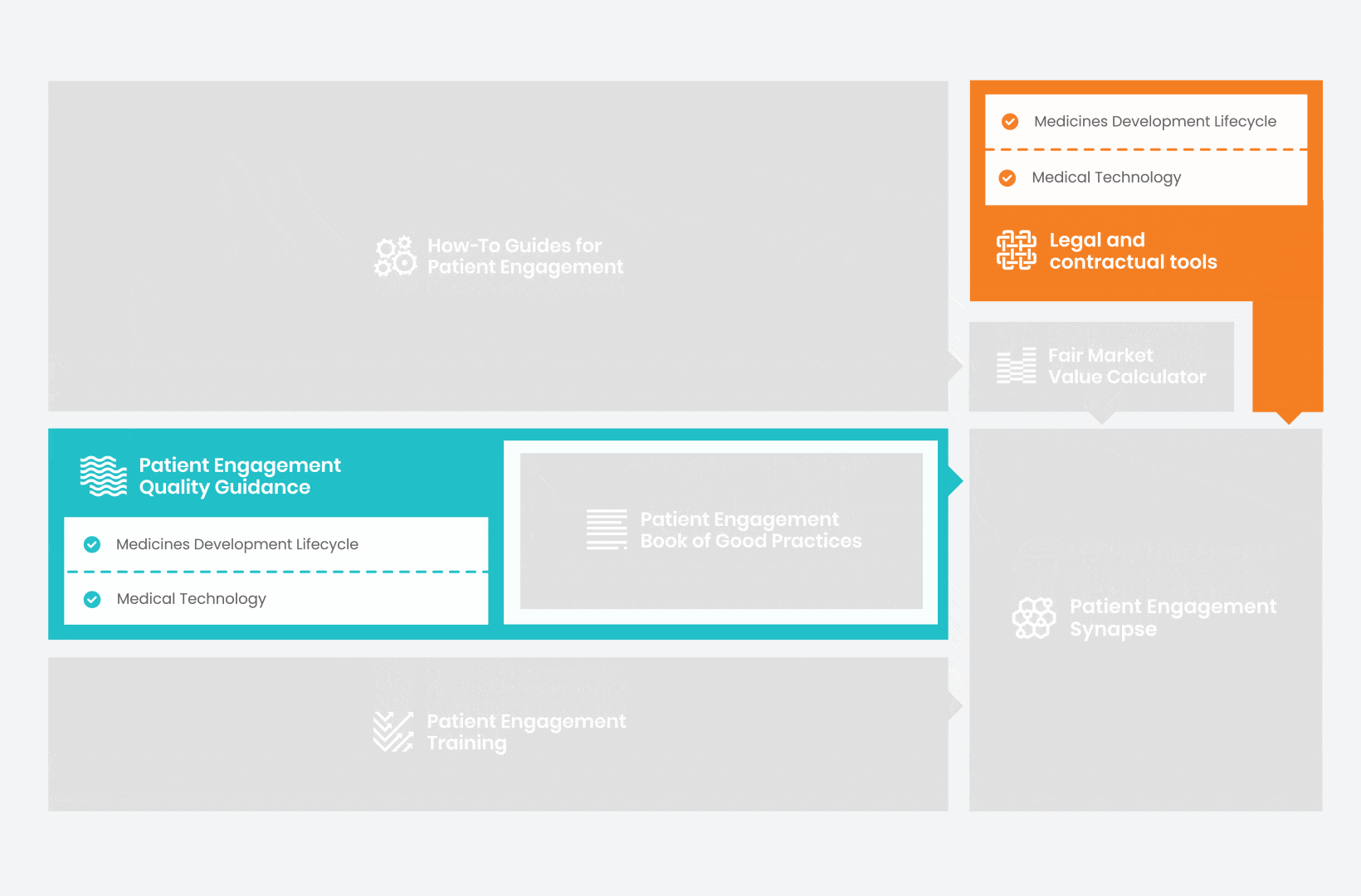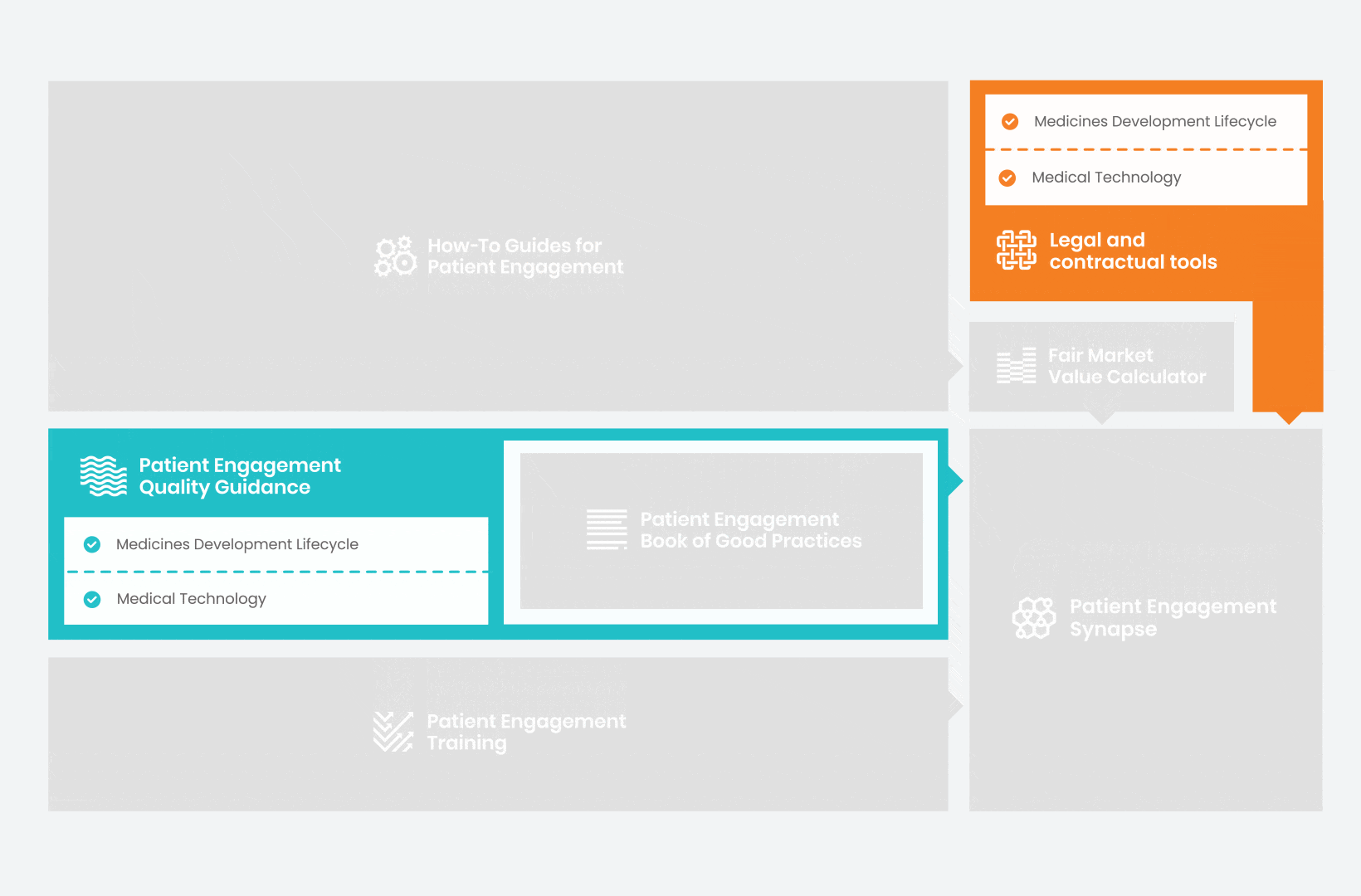
Therefore, over 70 experts from 51 organisations worked together to deliver the Patient Engagement Quality Guidance – a practical guide to planning,
developing and assessing the quality of patient engagement activities in medicines development or medtech projects.”
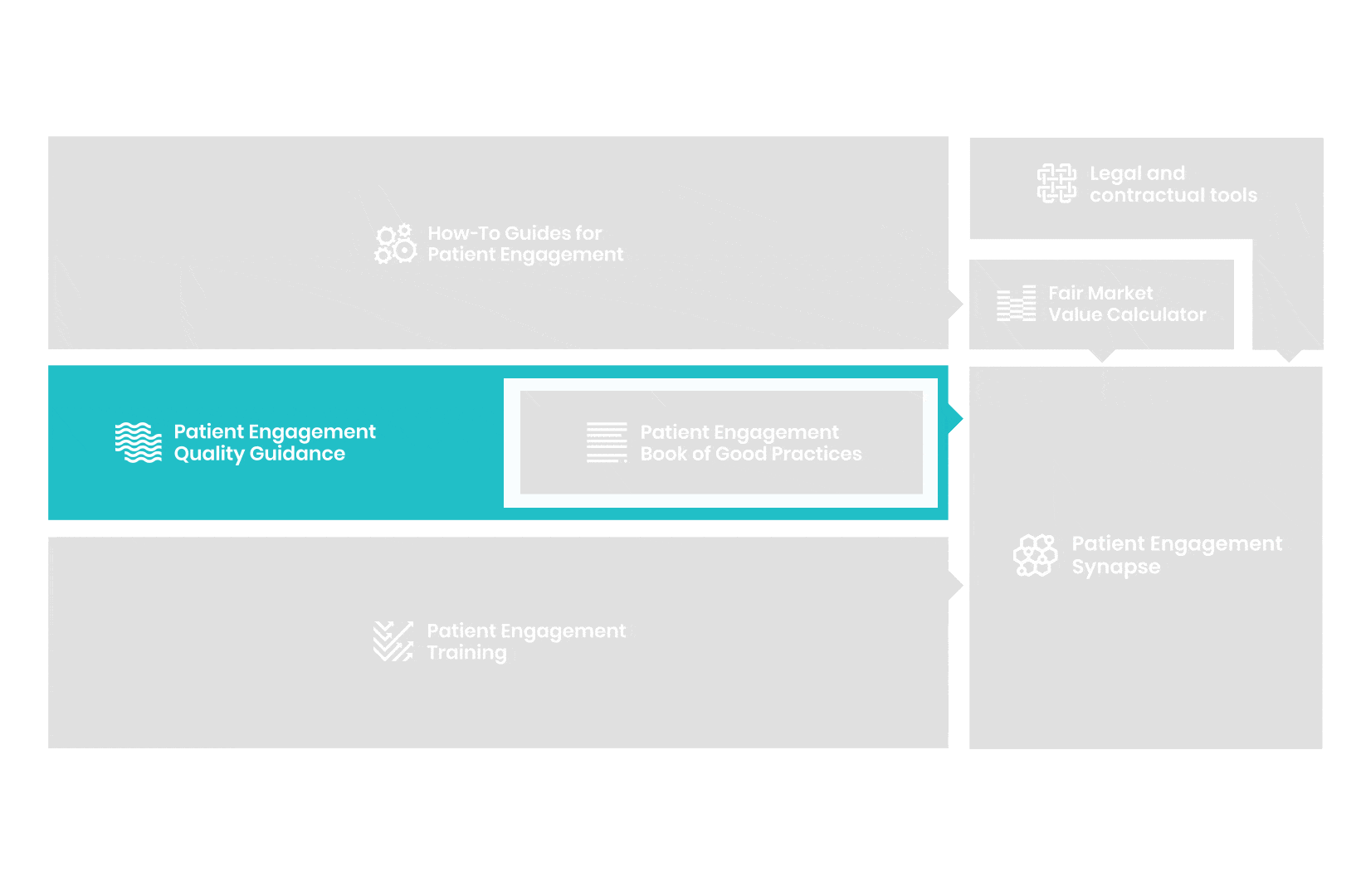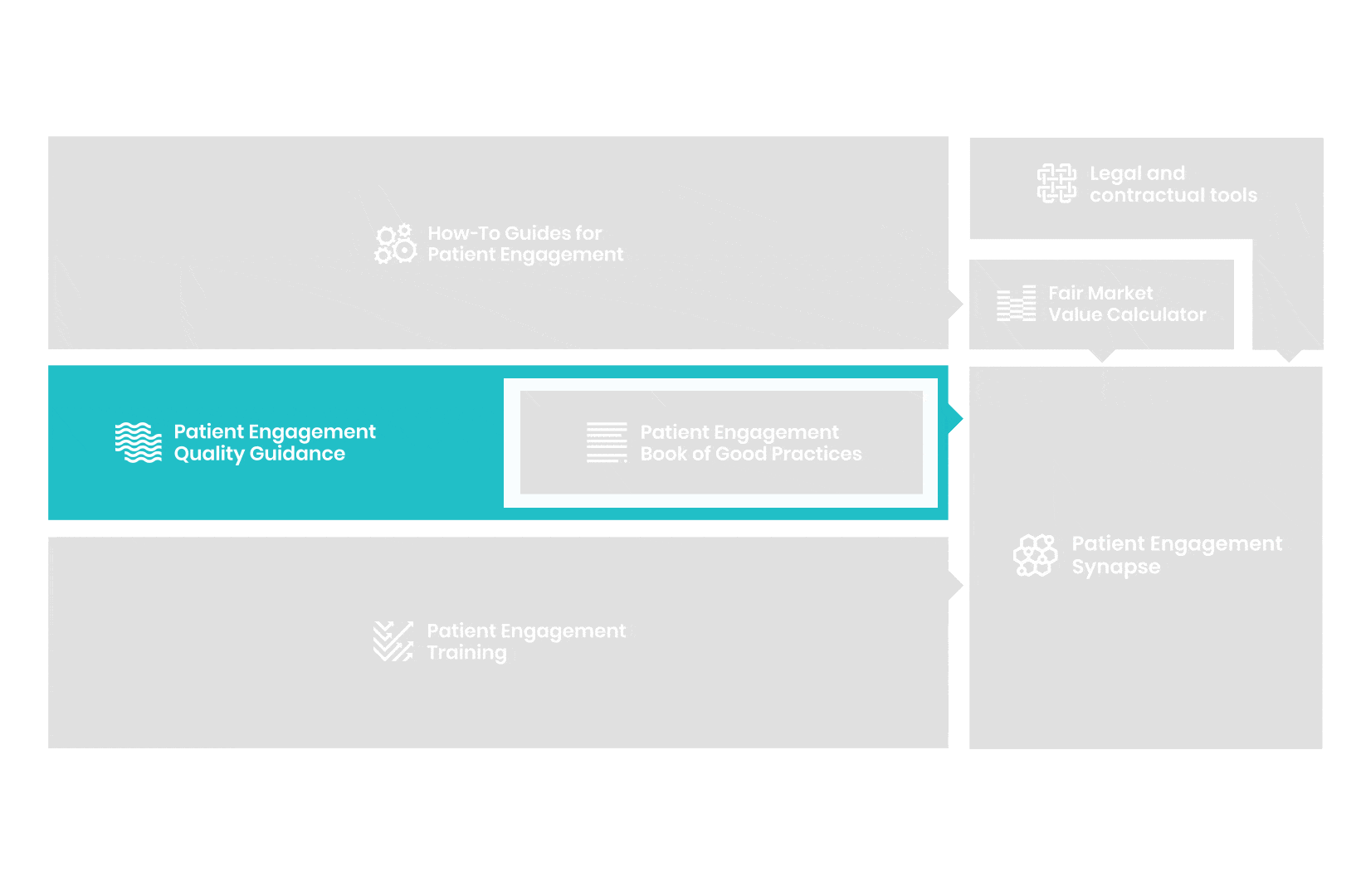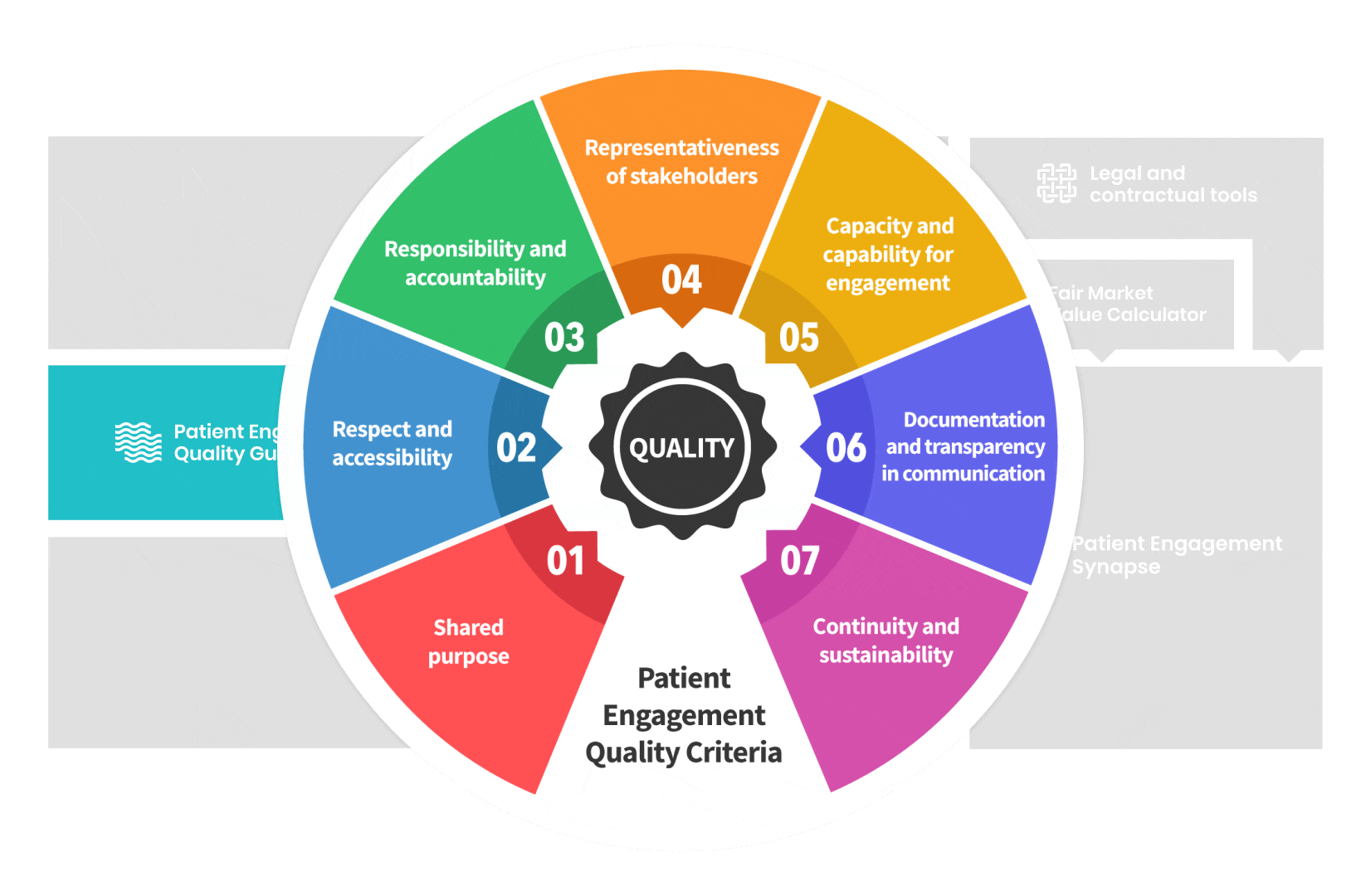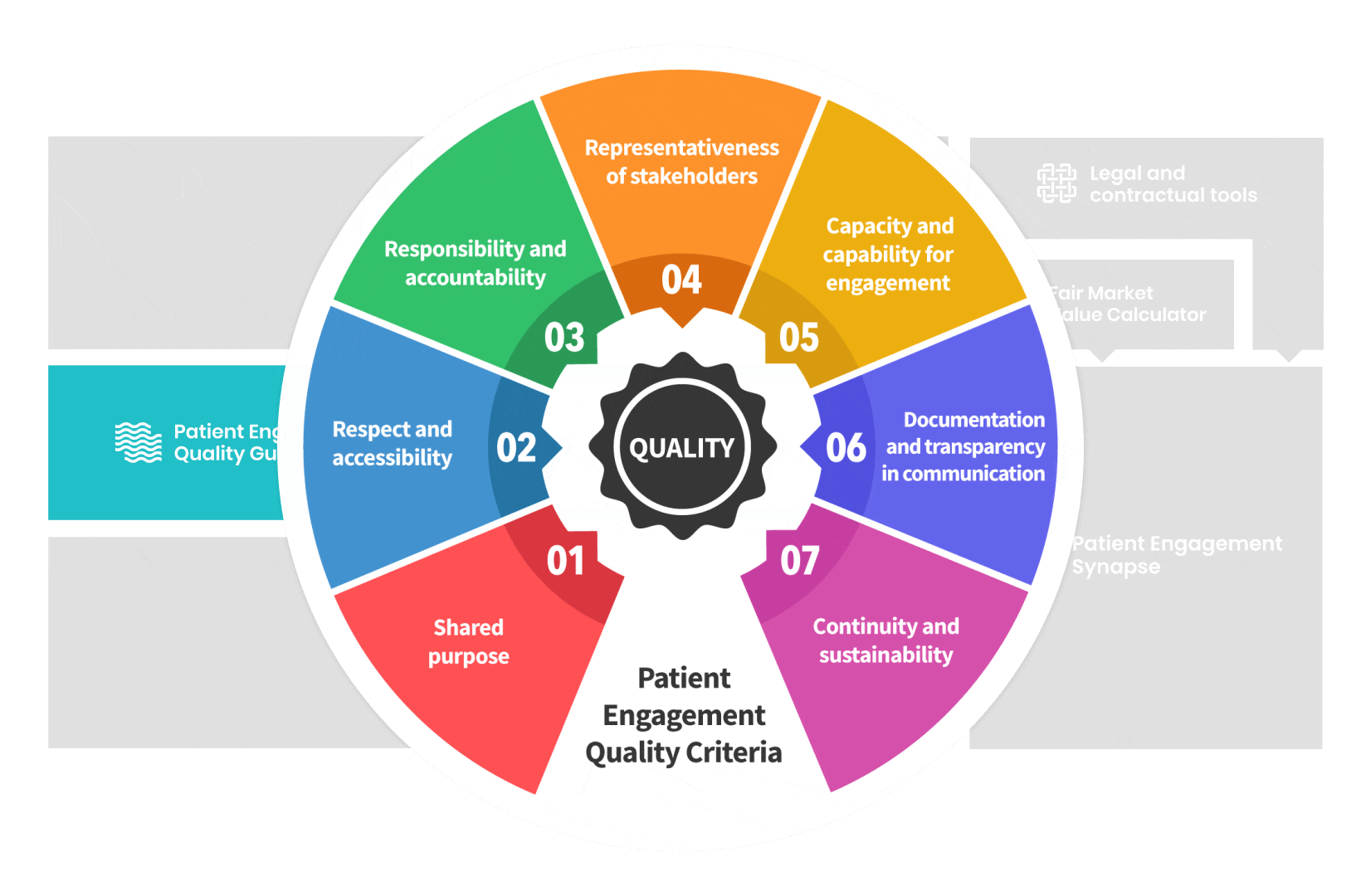
The guidance introduces seven Quality Criteria to assess patient engagement practices that have been consolidated
from published patient engagement frameworks and further co-developed by 7 working groups.
To complement the Patient Engagement Quality Guidance, a selection of patient engagement initiatives that can be considered positive real-world examples were consolidated into the Book of Good Practices.
The selected initiatives demonstrated two or more of the seven Quality Criteria, serving as an inspiration for those willing to involve patients. Each year a new edition is being published.
During the landscape and needs analysis, over 150+ activities recommending patient engagement were carefully extracted.
In 2018, working groups consolidated these 150+ activities into a Public Consultation seeking expert opinion to support the development of guidance for the prioritised activities.
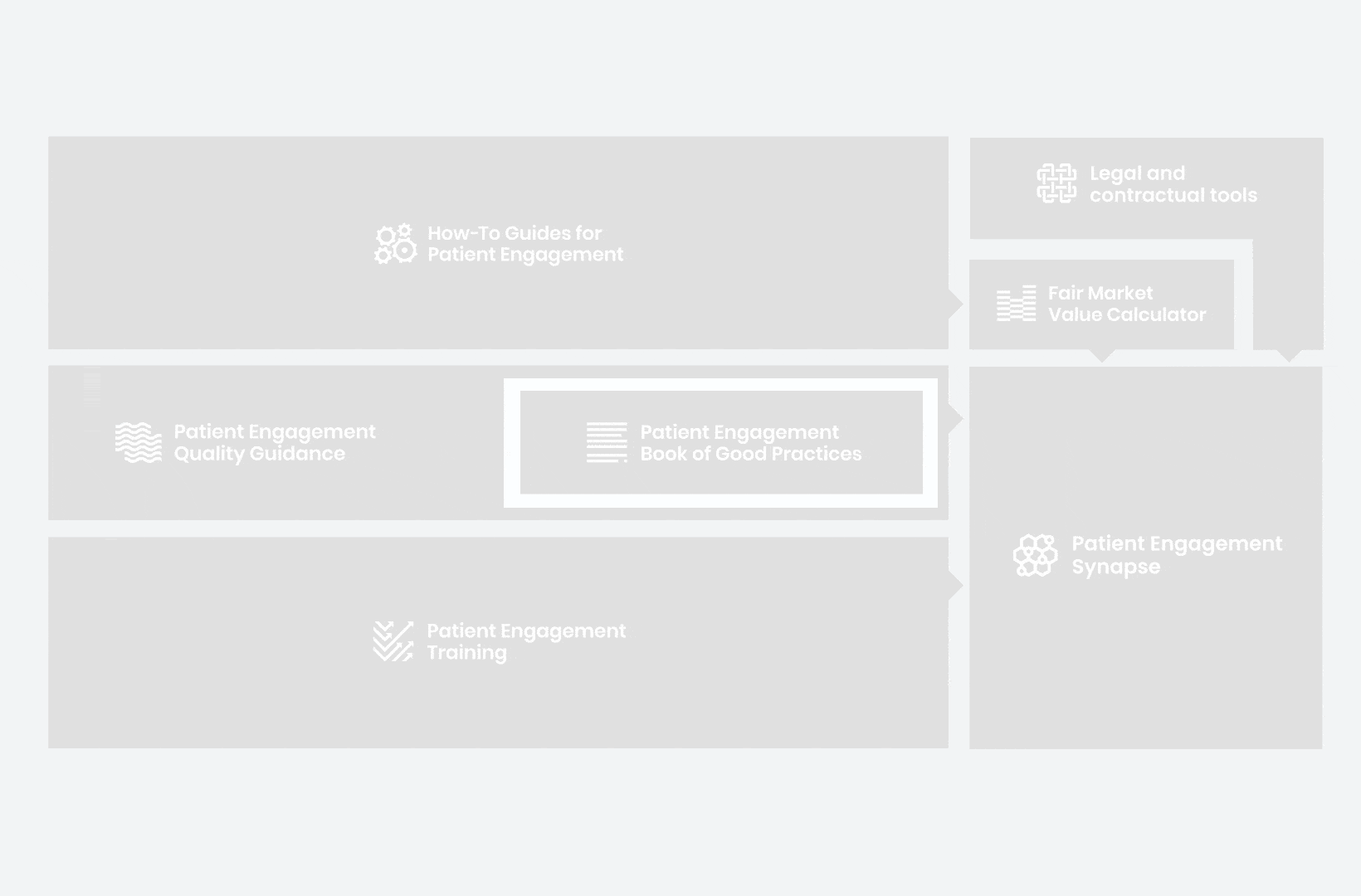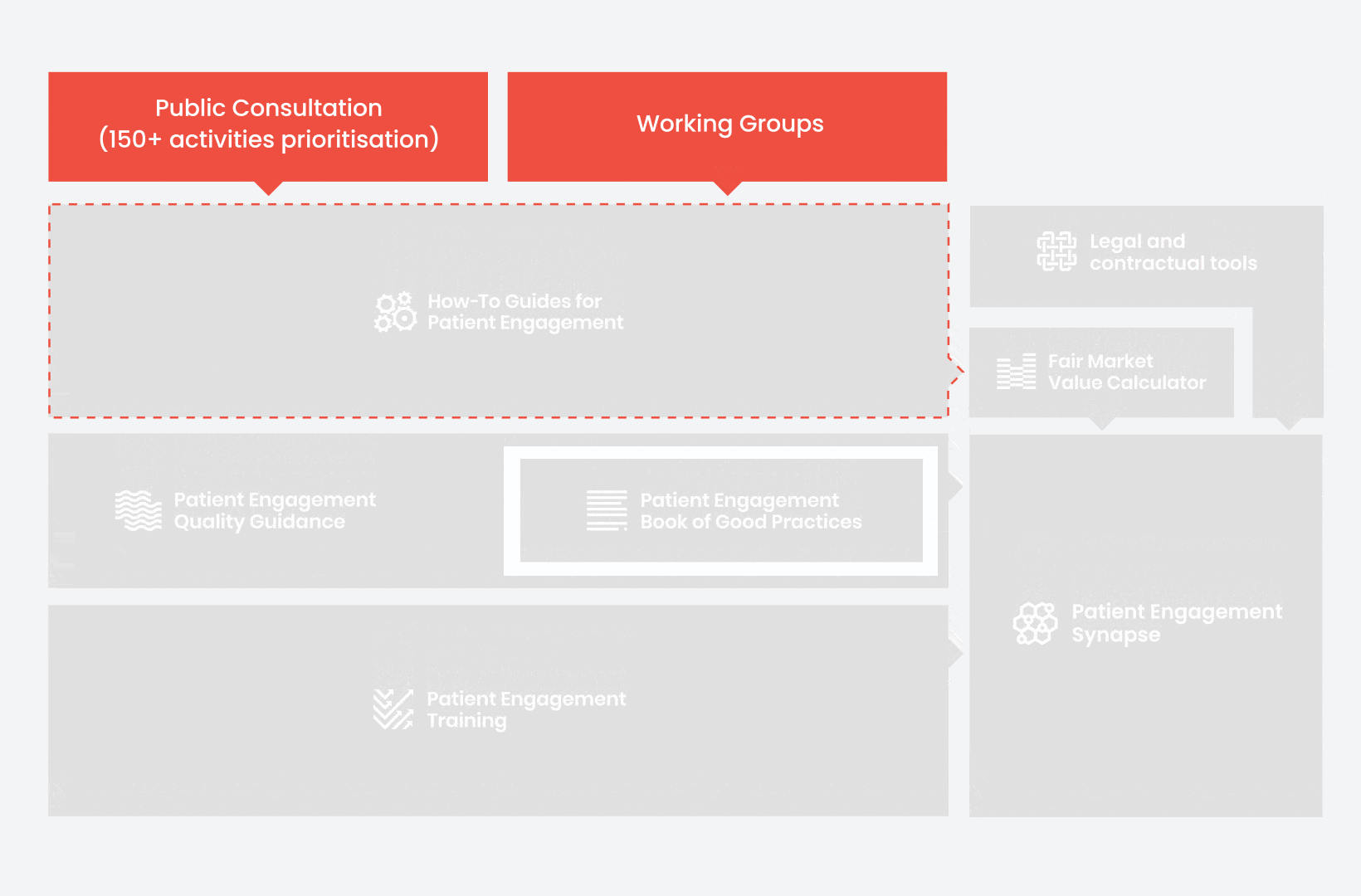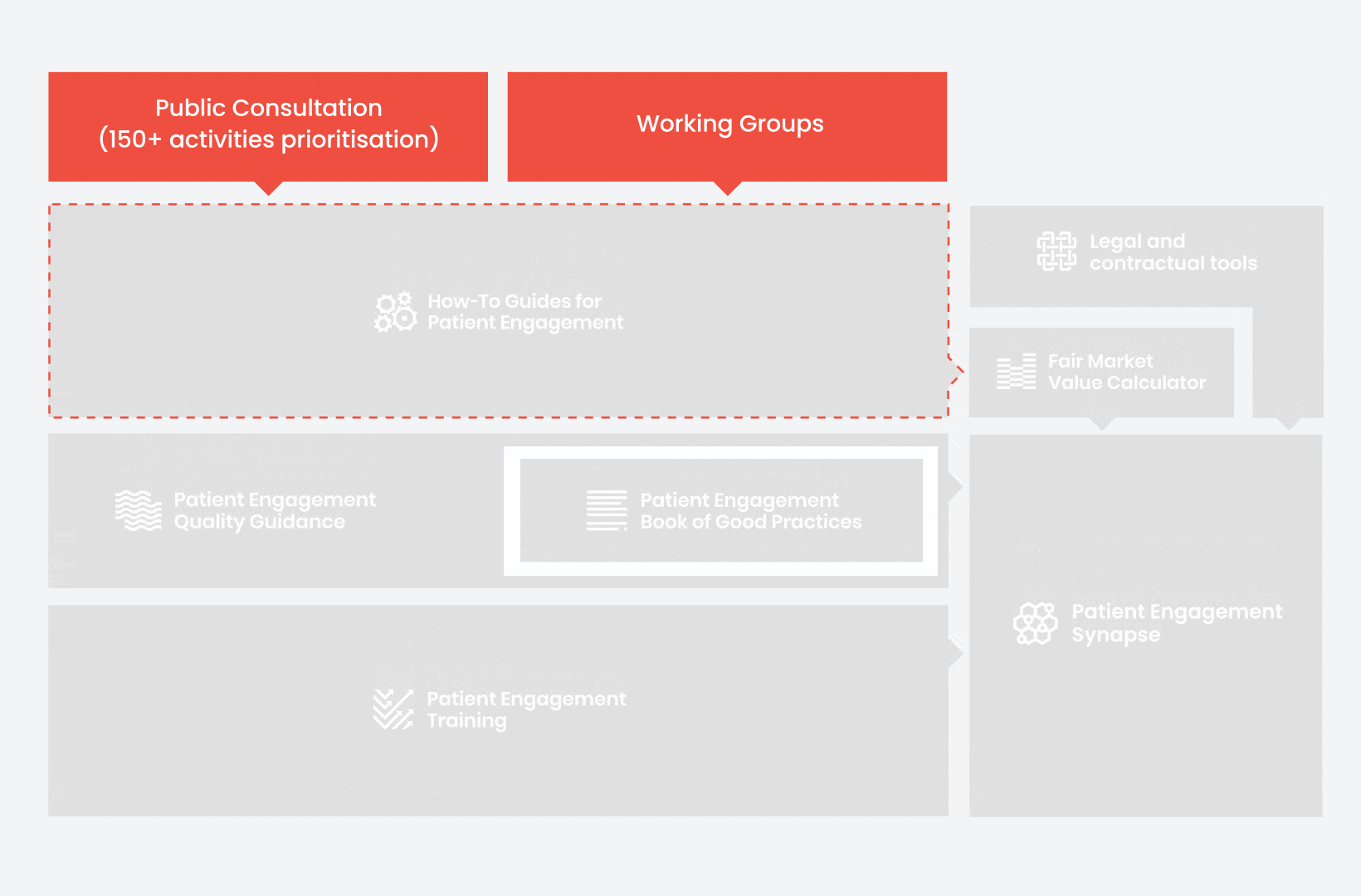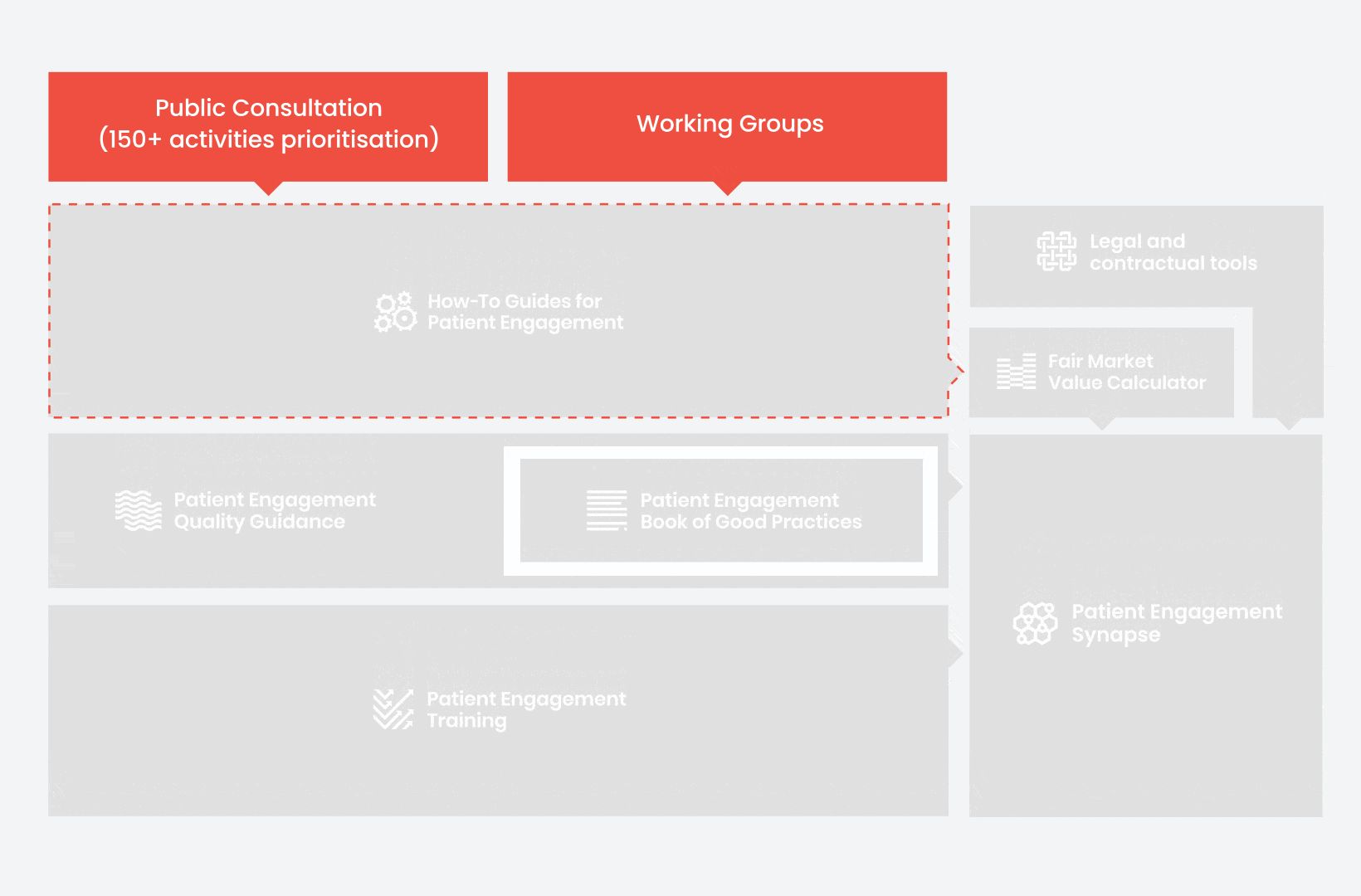
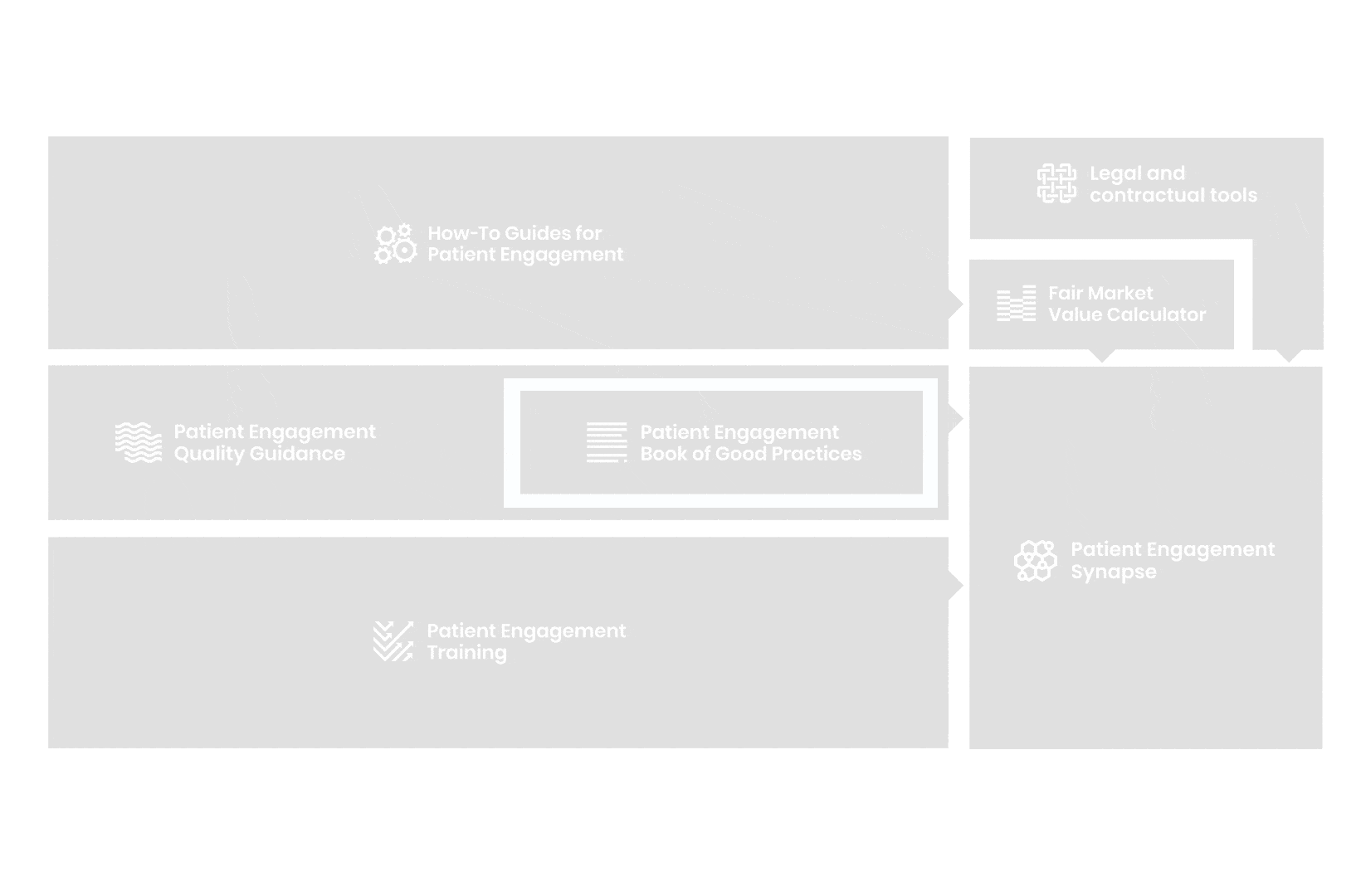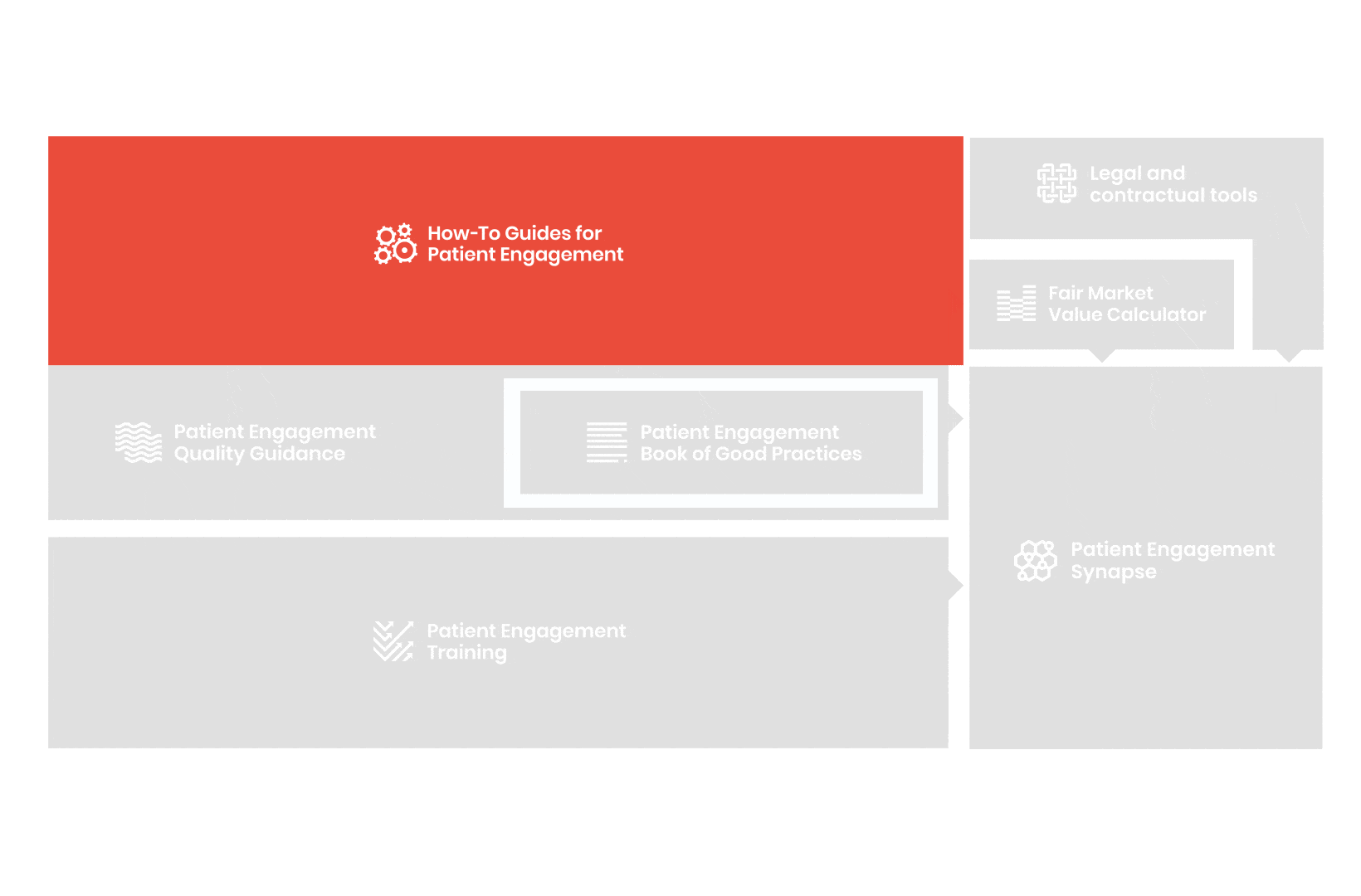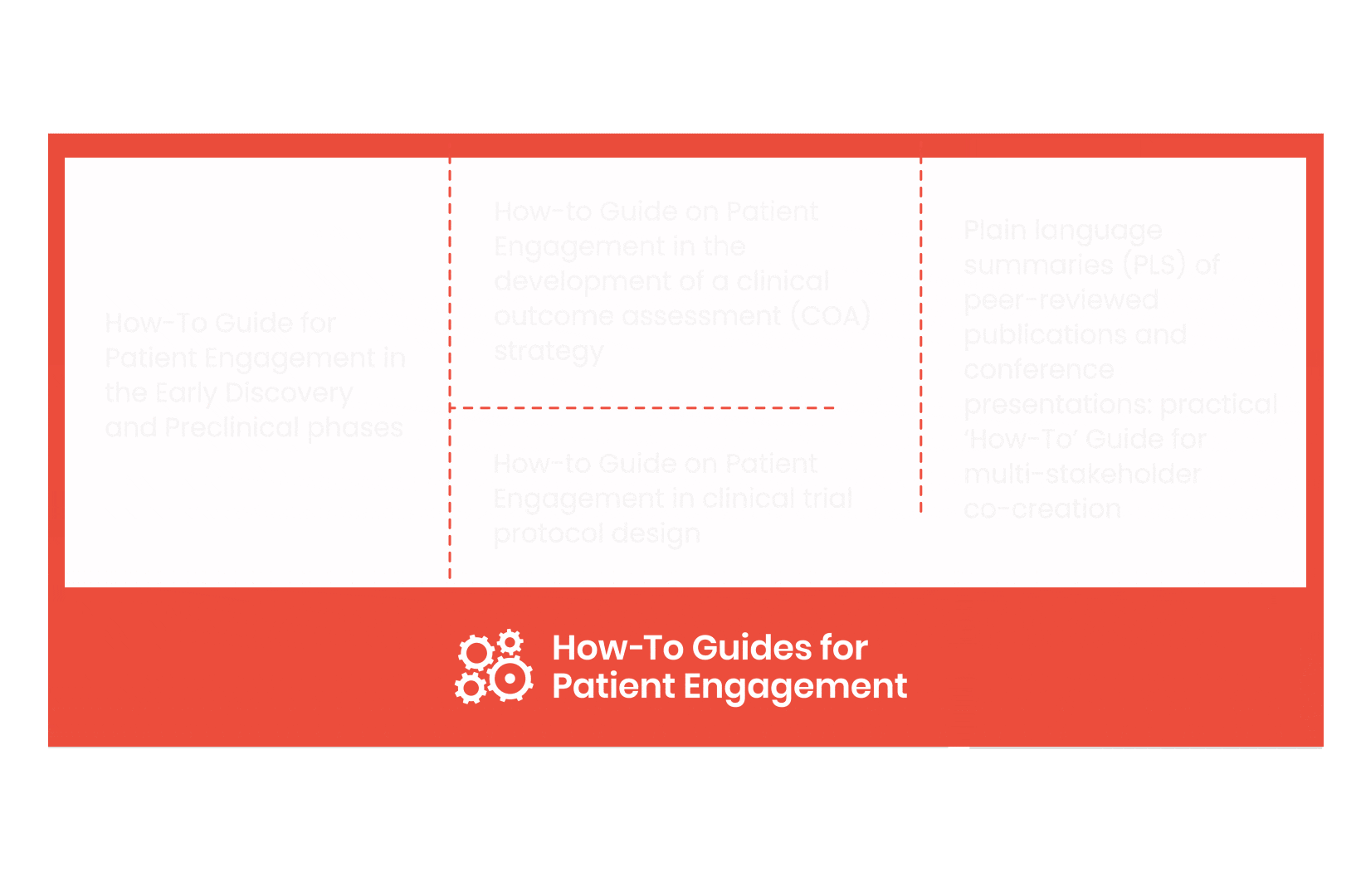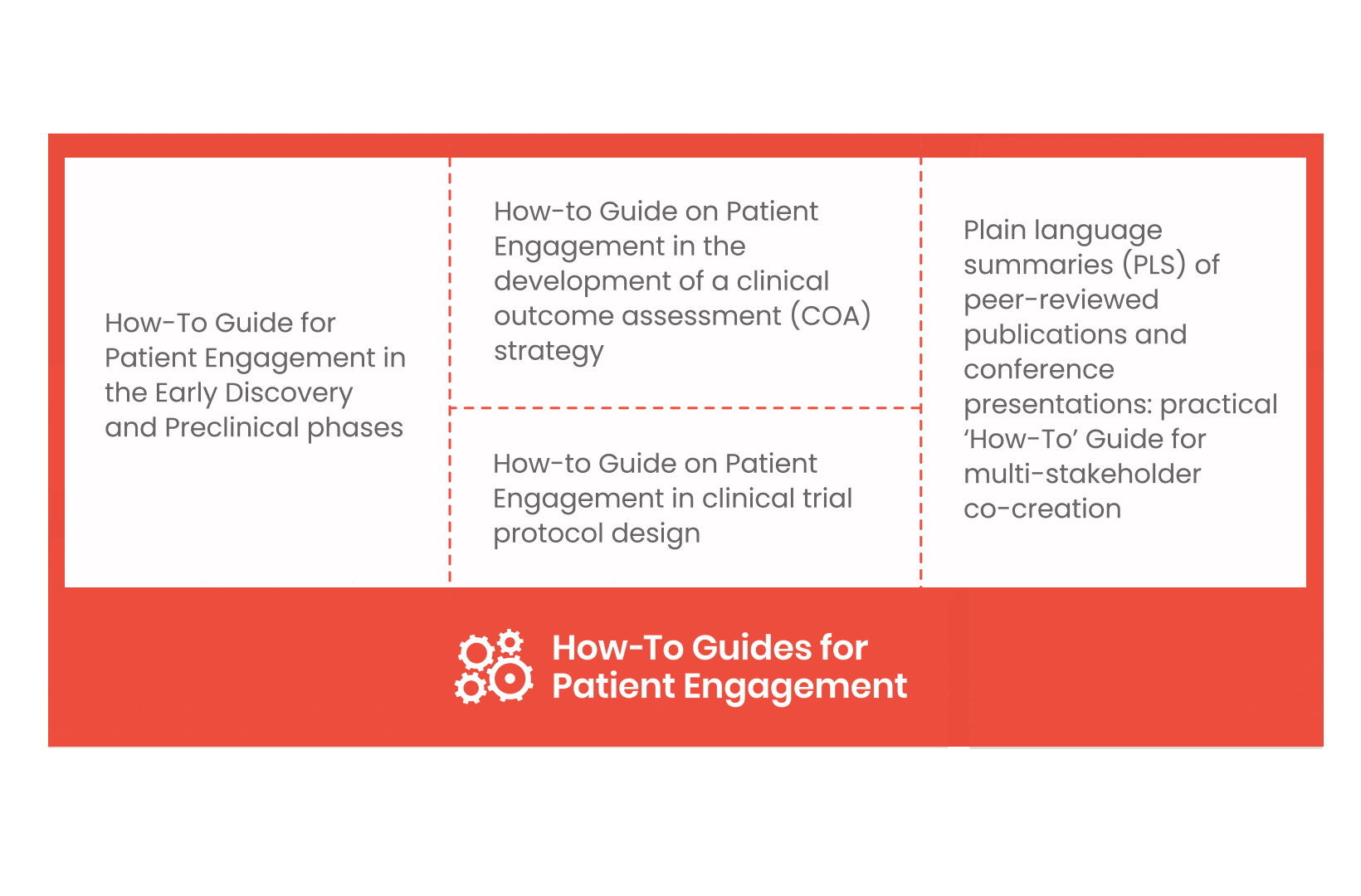
From there, co-creation working groups started to take shape, aiming to develop co-created How-To Guides covering the previously prioritized activities.
These would be specific activities from the medicines development phases but also covering overarching topics (i.e. plain language summaries, capacity building etc). Find out more about the How-to Guides here.
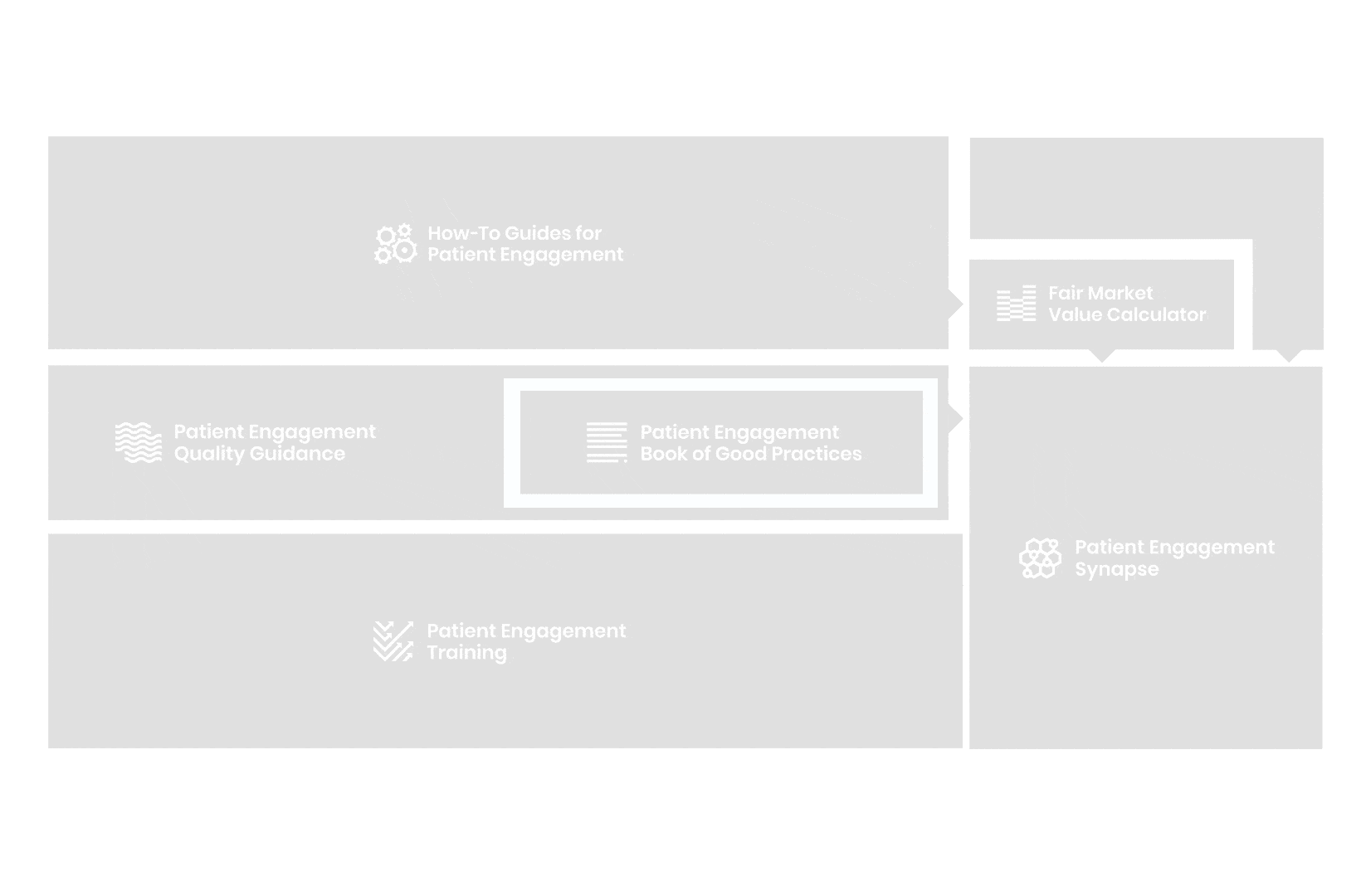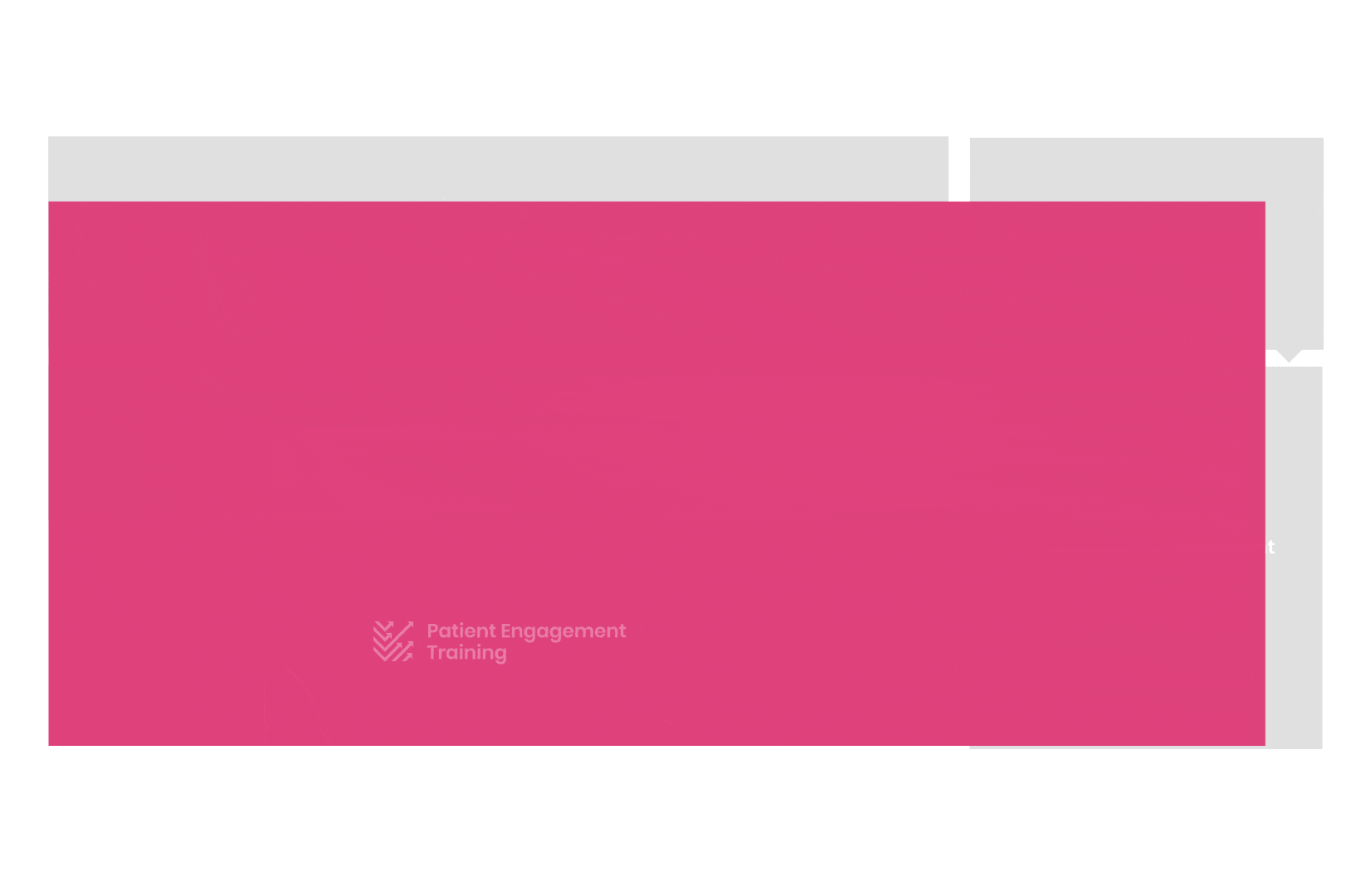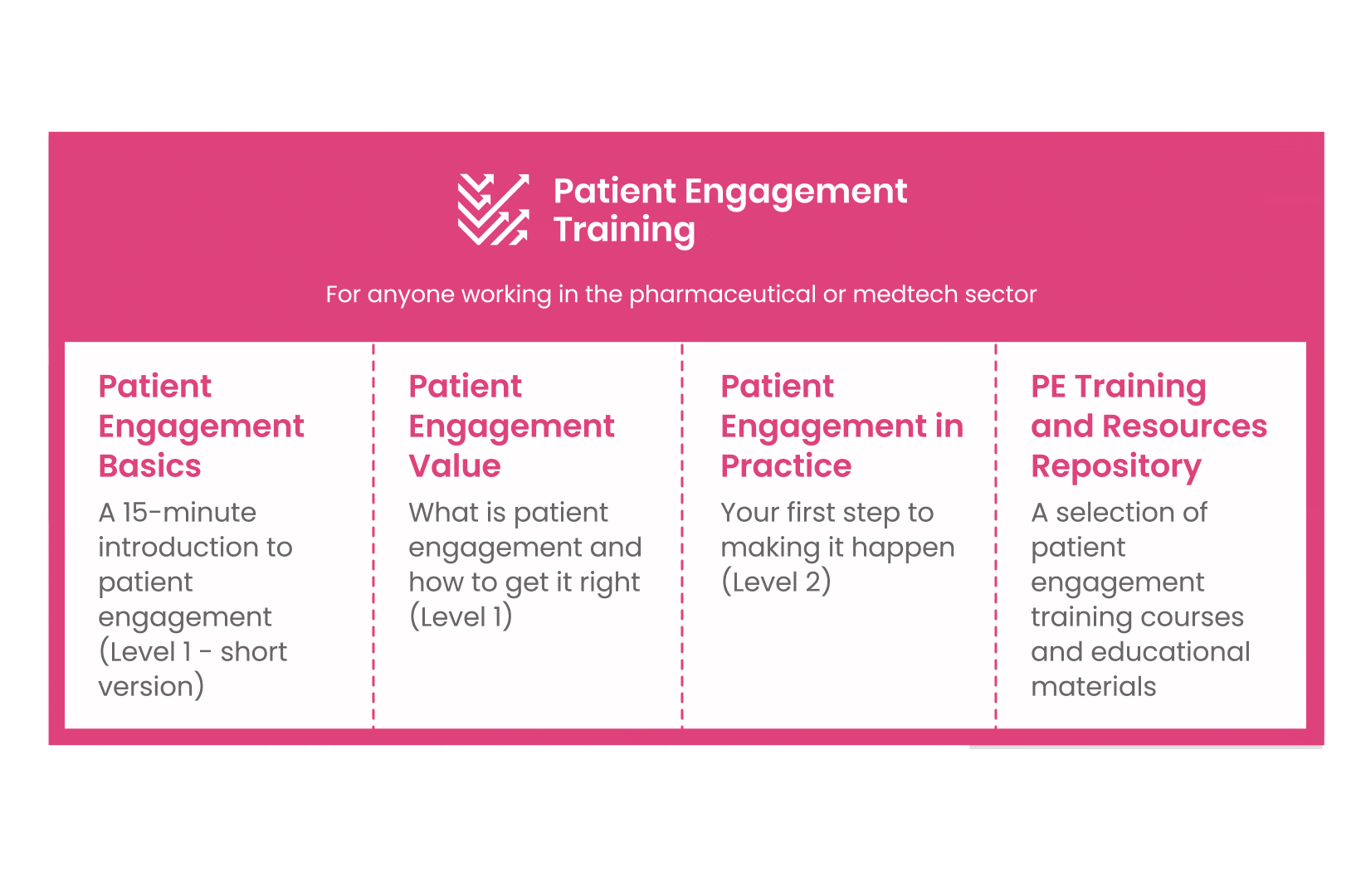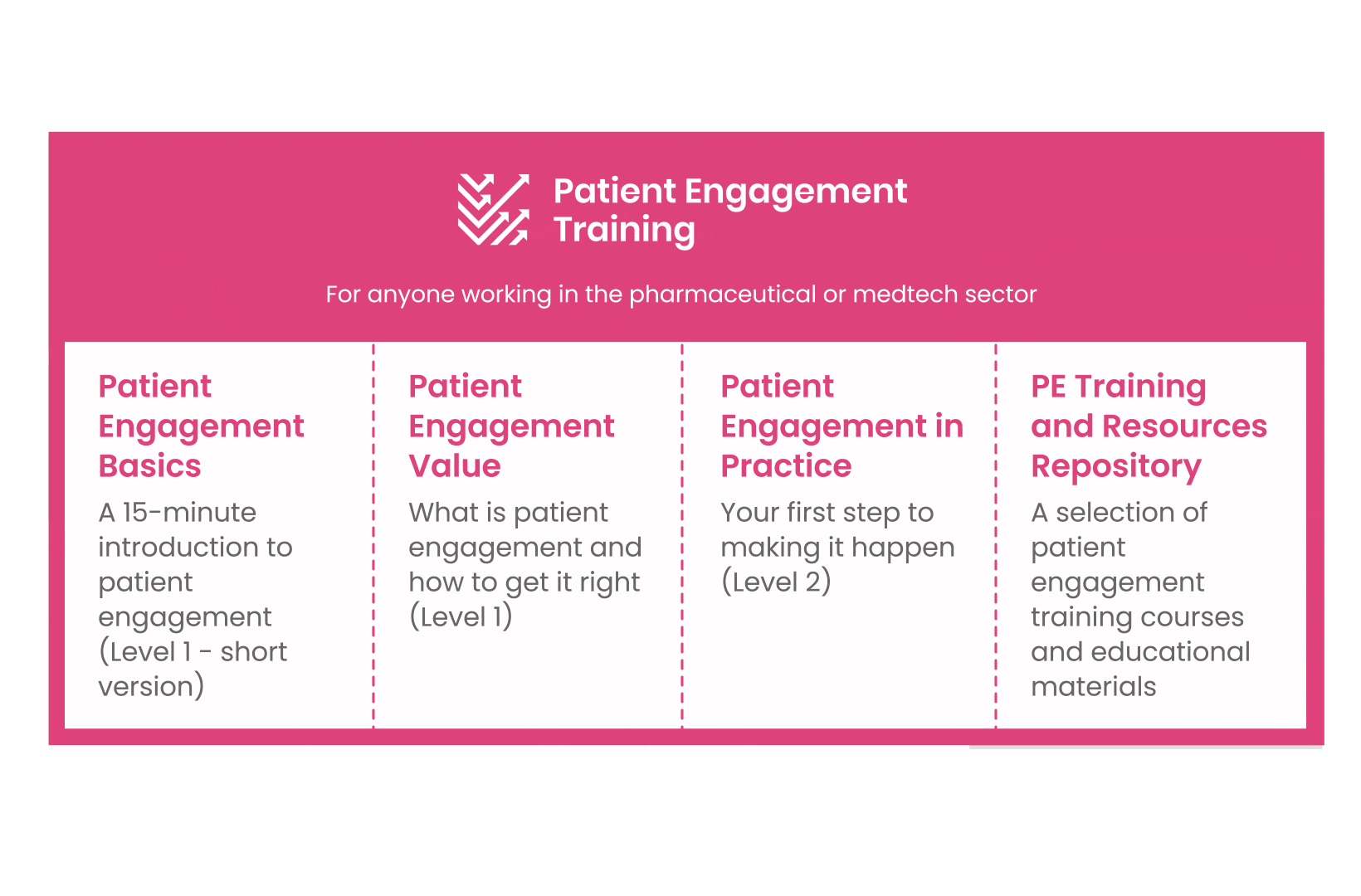
But this was not enough. In the summer of 2018 a common need was identified for a co-created, unified capacity-building effort. Thus, the Patient Engagement Training effort was initiated by 6 pharma companies,
working together WITH patient organisations and regulatory bodies to deliver a comprehensive, practical and cohesive training program for anyone looking to start or advance in their patient engagement journey. You can take the training here.
In parallel with this work, a patient engagement hot topic was being discussed at length – remuneration for patient experts at fair market value.
Therefore, The National Health Council (NHC), PFMD and many other partners started the work of developing a tool that would assist stakeholders to confidently enter into ongoing, compliant, and sustainable engagement – The Fair Market Value Calculator.
The Calculator is complementing the Legal Agreements initiative – a collaboration with WECAN, MPE and over 10 pharmaceutical companies.
The co-creation process in this initiative saw the launch of four Reference Agreements which could be considered the starting point for all patient engagement endeavours.
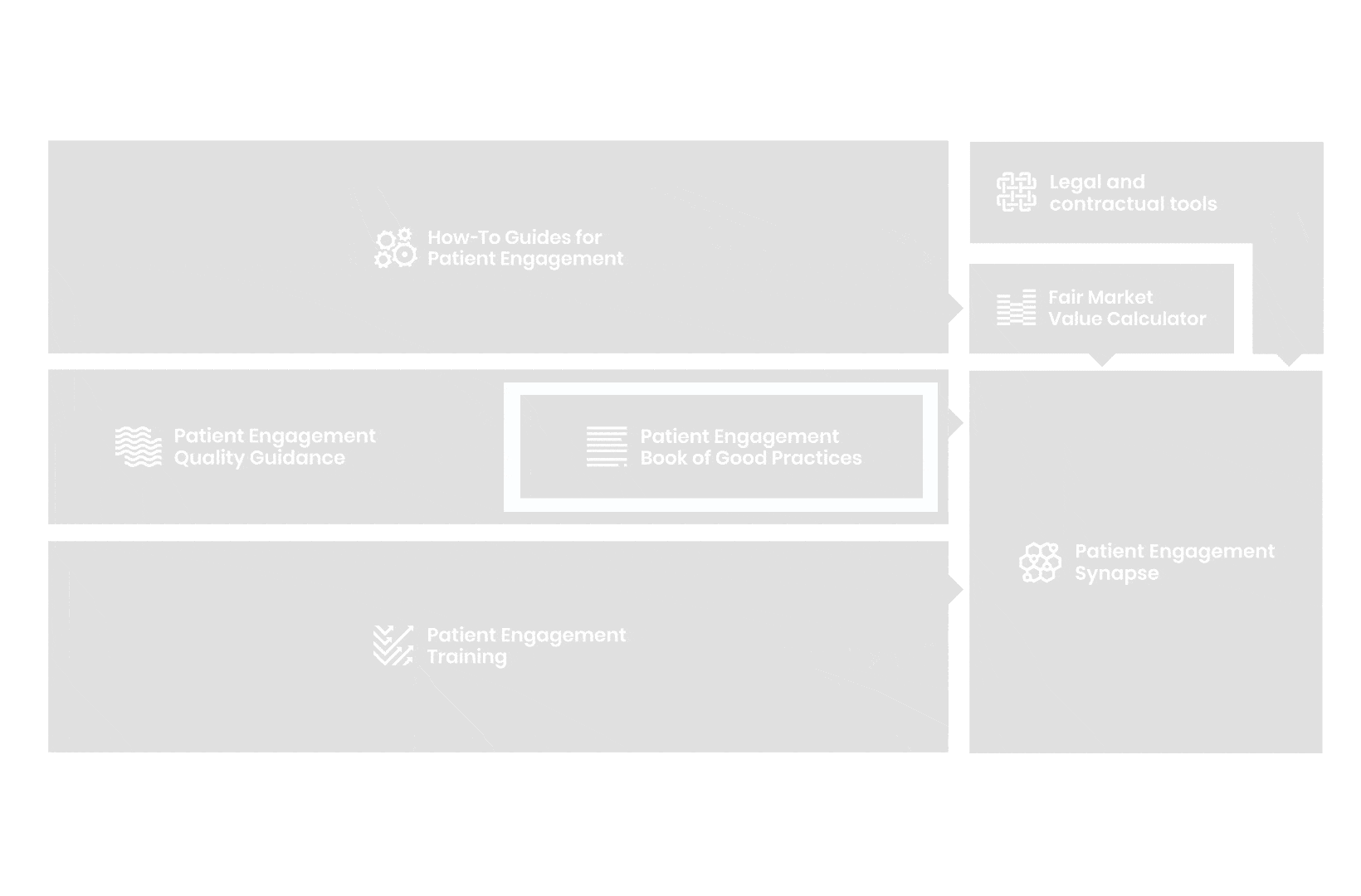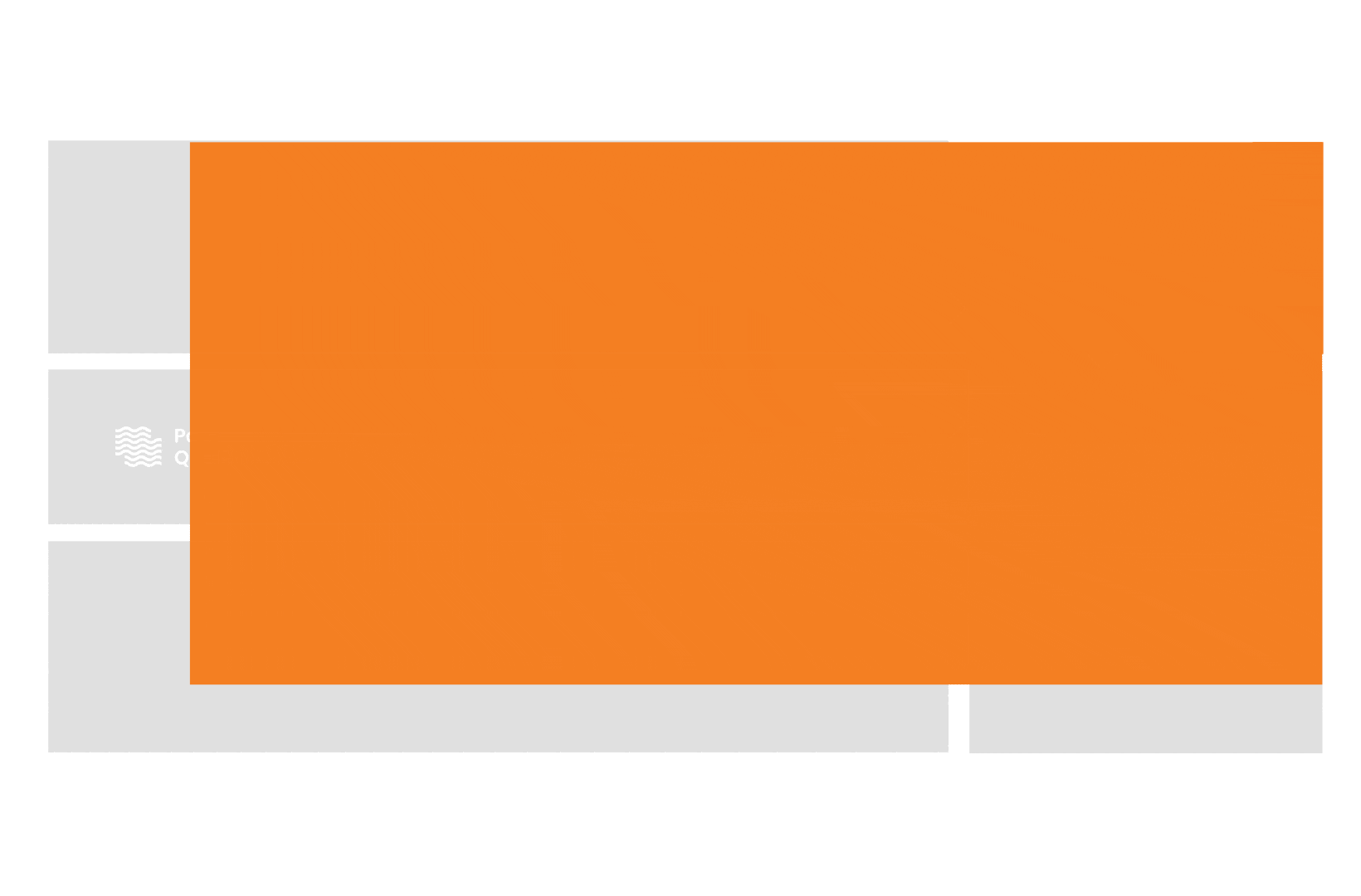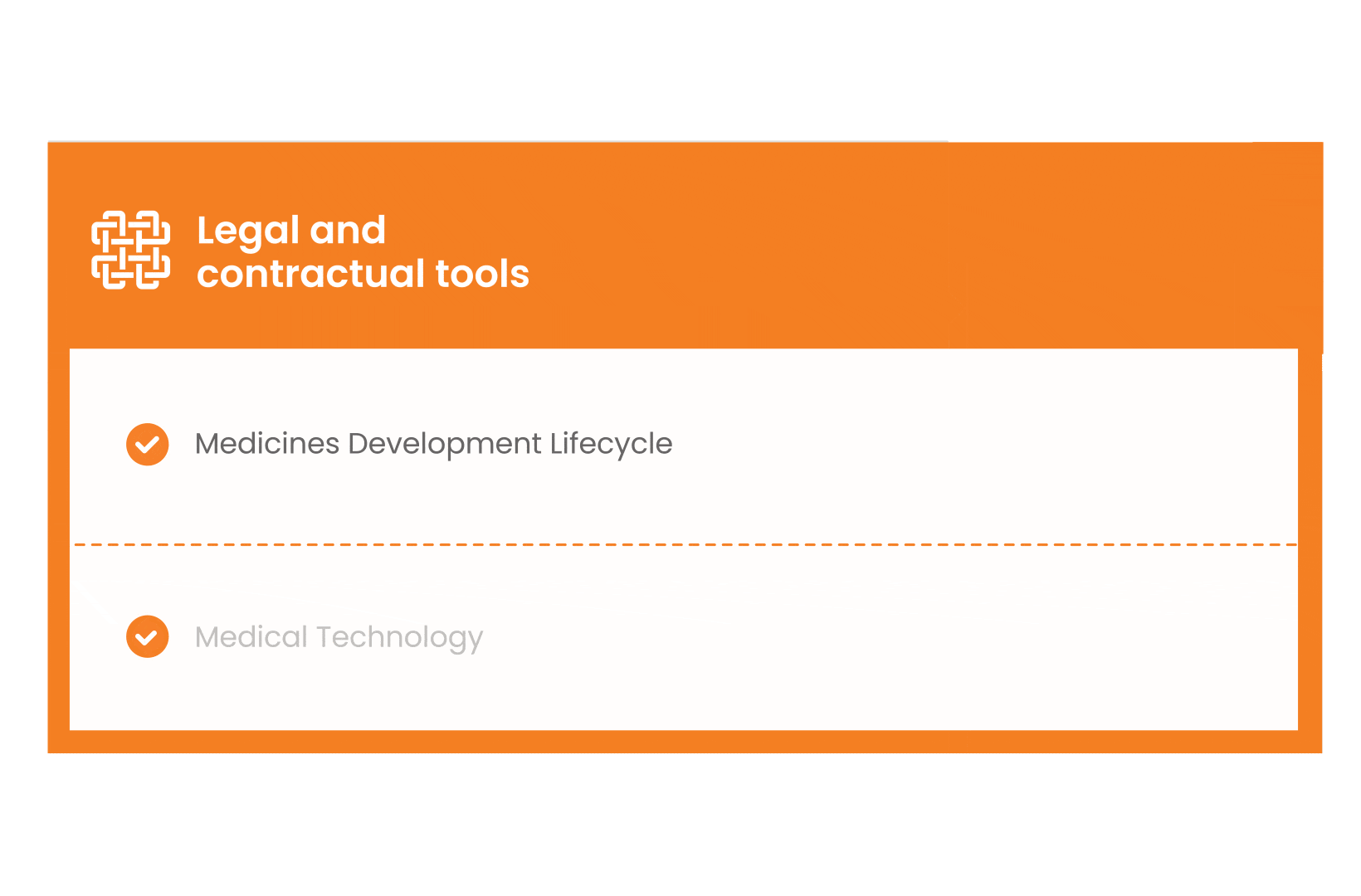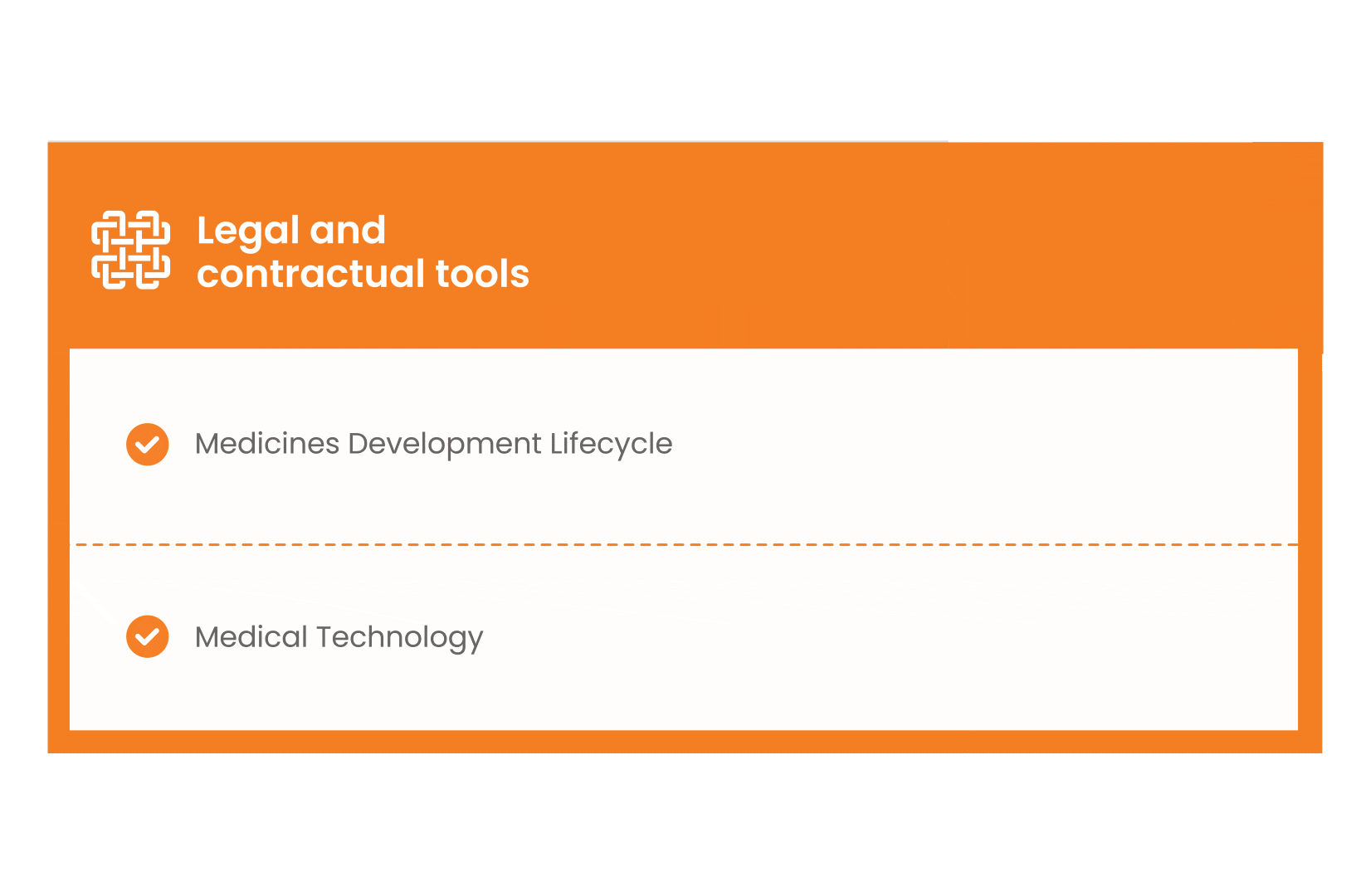
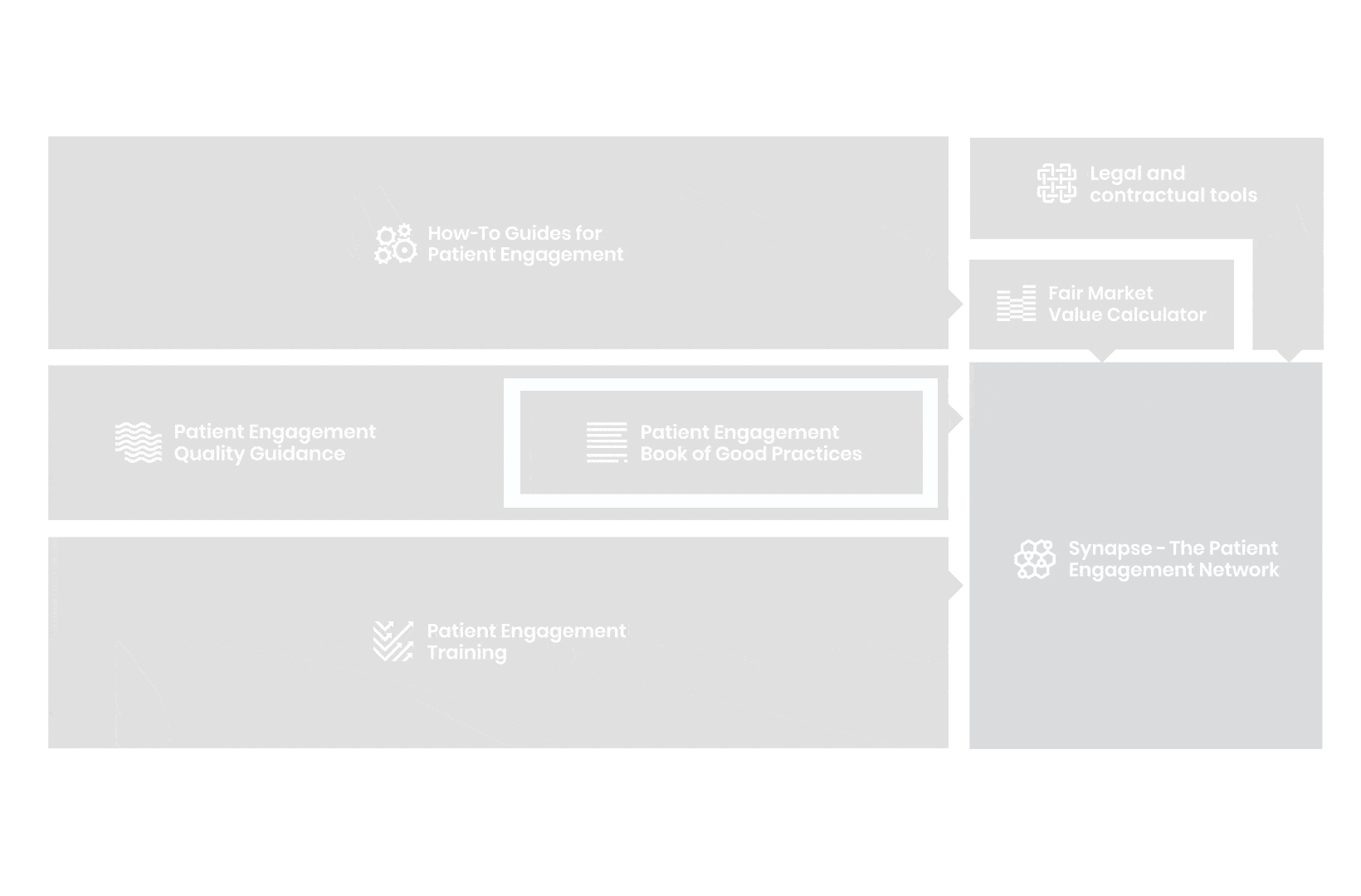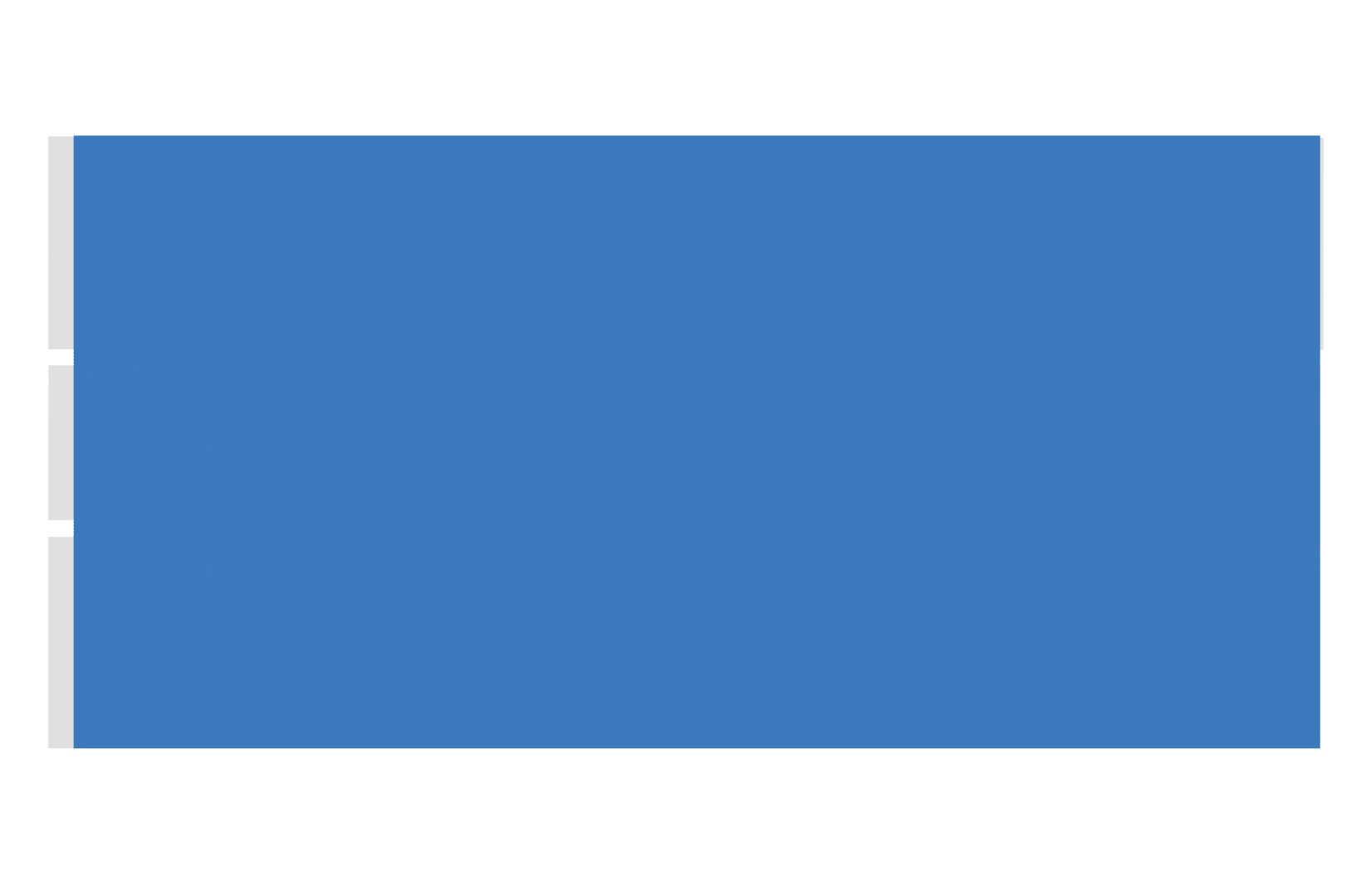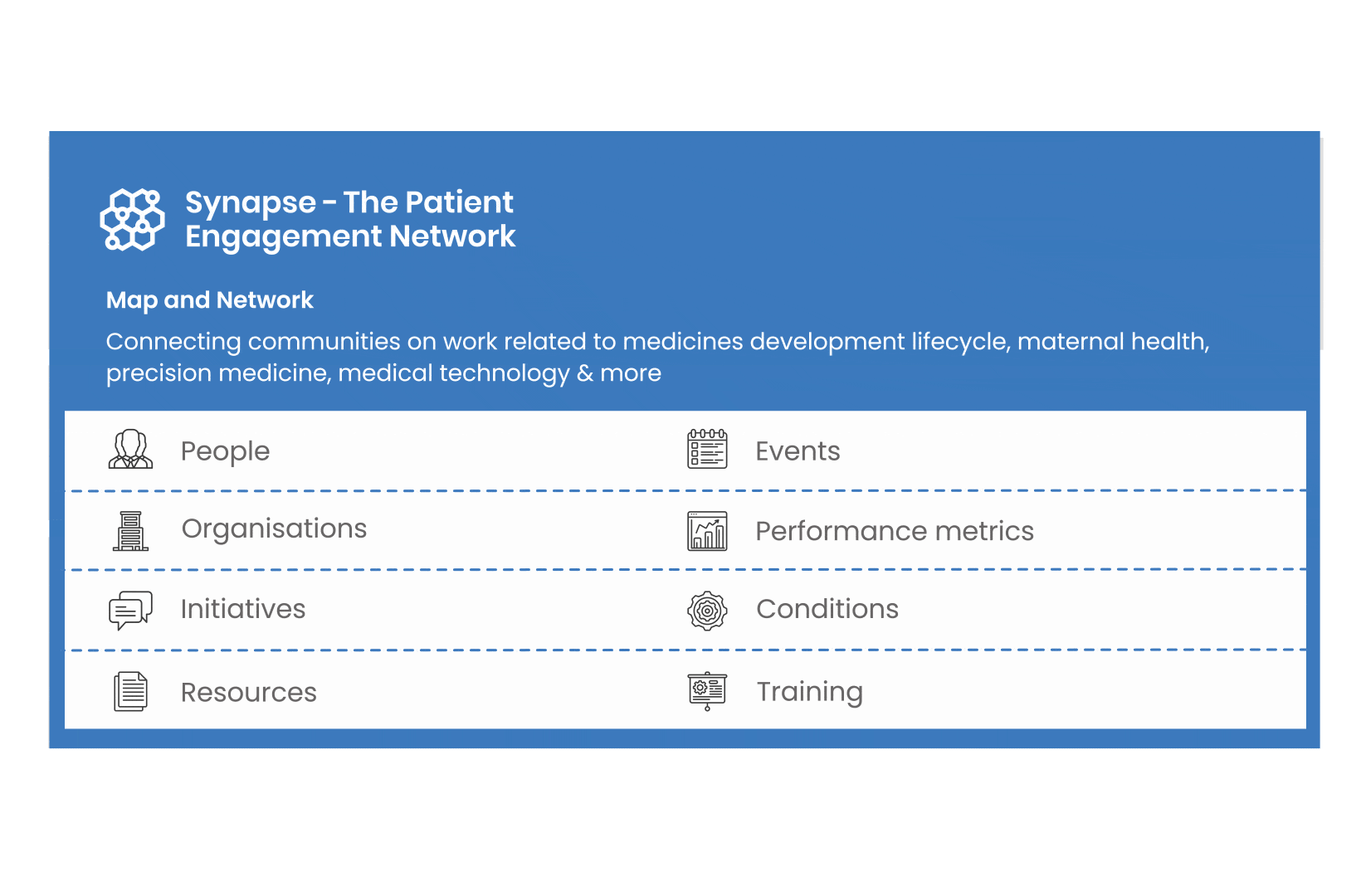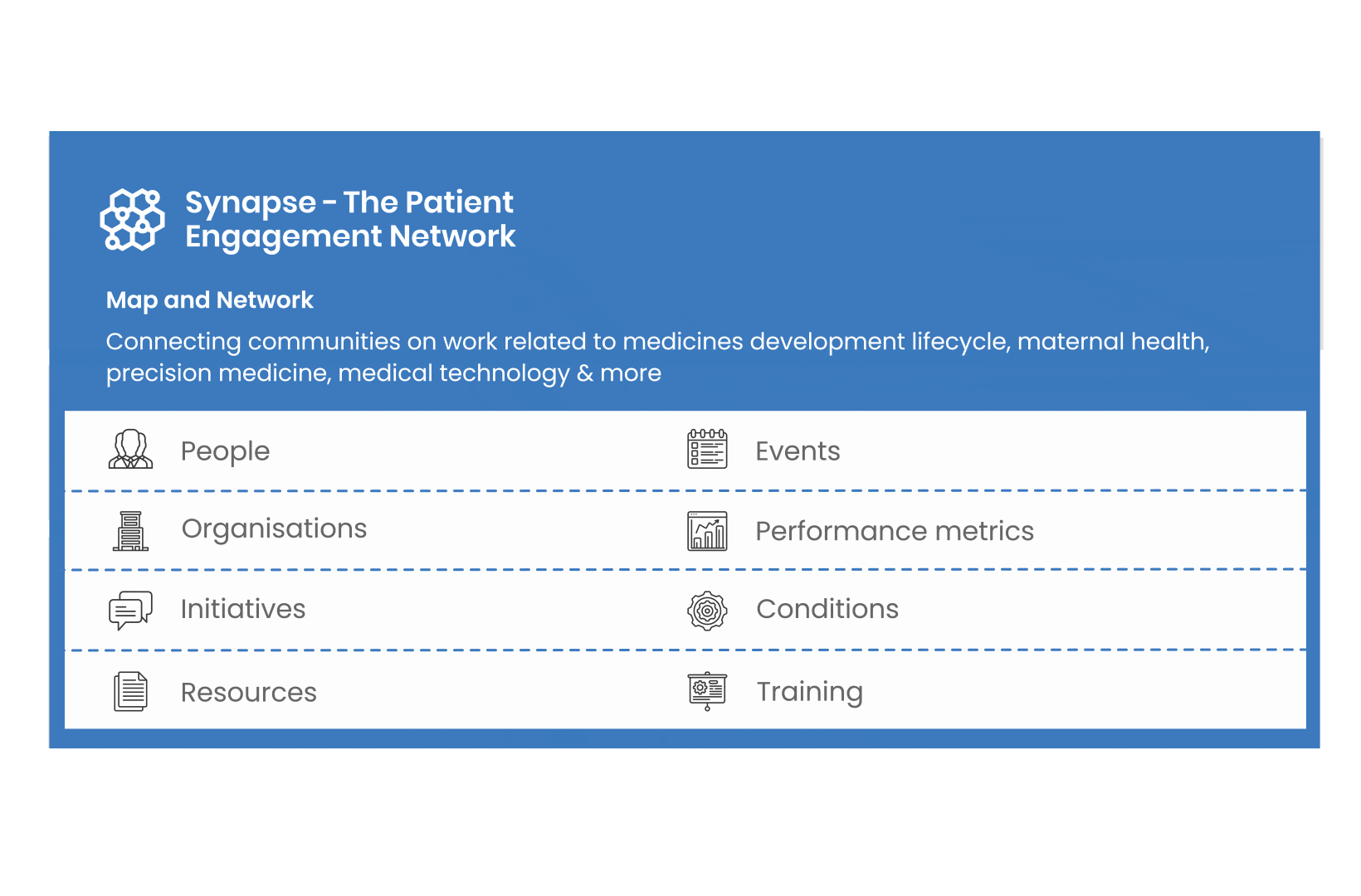
All these integrated tools are showcased and integrated with Synapse – a digital solution to empower all players,
by mapping and connecting experts, organisations, events, initiatives and resources of the patient engagement world, bringing to life its whole related ecosystem.
A multi-stakeholder group consisting of 14 organizations from the medical device industry and patient organizations came together in 2020
to adapt several PFMD co-created Patient Engagement tools, to ensure their suitability and applicability for the medical device industry.
As part of the PFMD commitment to PARADIGM sustainability, several PARADIGM-developed tools have been reviewed and integrated in the PEM Suite framework.
More improvements will follow