Co-creating a meta-framework for patient engagement takes collective effort and commitment. We have now held 5 workshops with 62 international participants representing patients/ patient organisations, industry (including CRO and biotech), independent experts (with backgrounds from academia, research and also industry), HTA and regulatory.
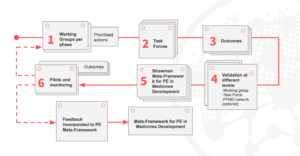
The Working Groups established in 2016 have been further divided into more agile task forces which have different focuses of completing prioritised action points from the action plans of the Working Groups.
The currently ongoing task forces focus on creating a Book of Good Practices from early research to post-launch and post-marketing phases, and tackling the compliance and legal barriers in a truly co-creation spirit. The work is being done individually as well as in one-day workshops, where task forces discuss and consolidate their findings.
Working Group methodology
Watch the space for more information or contact us if you want to get involved.
Co-creation through collaboration – PFMD task forces
Tags:

































































