Nicholas Brooke, Patient Focused Medicines Development Executive Director, talks about the HTAi conference in Vancouver which illustrated the benefit of deploying robust patient-engagement methodologies early in the medicines development process.
Nicholas Brooke, Patient Focused Medicines Development Executive Director, talks about the HTAi conference in Vancouver which illustrated the benefit of deploying robust patient-engagement methodologies early in the medicines development process.
Health Technology Assessment (HTA) explores the value of a new health technologies compared to existing products. Incorporating patient voices in this process is essential. However, HTA can be complicated and professionals in the field may be naturally reluctant to add a new layer of complexity.
At the HTAi conference in Vancouver, the topic was debated during a plenary session which illustrated a broad interest in patient engagement as well as some of the perceived barriers to making it a reality. The discussion focused on the burden of patient engagement on the HTA community, the current and growing fragmentation of patient engagement methodologies across HTA bodies, and the need to capacity development among the patient community as well as among health professionals.
Patient Focused Medicines Development Executive Director, Nicholas Brooke, who spoke at the closing plenary session, says fears about the burden of patient engagement are misplaced.
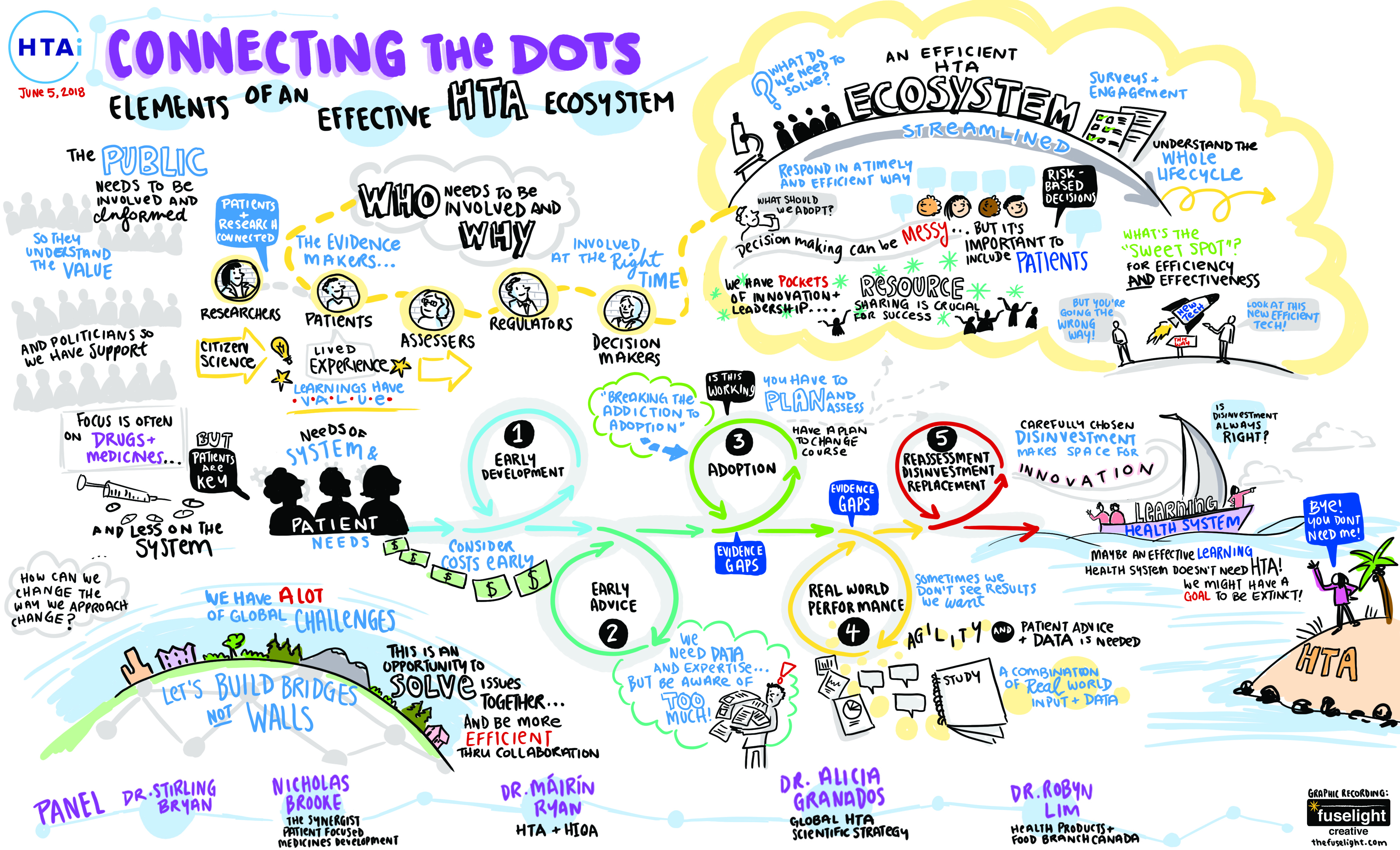
‘The solution is to streamline patient engagement across the whole lifecycle of drug development,’ he says. ‘There is still work to do in terms of implementing a shared methodology and training patients and health professionals. But if we incorporate patient engagement early, the overall burden on the health system is lower – and the results are better.’
By taking a whole-system approach, patient engagement work done during the early stages of medicines development can benefit HTA professionals. ‘It’s not a HTA problem; it’s a drug development problem,’ said Brooke. ‘If patient engagement is done right from the beginning,
HTA can work with that data. The key is to think about the whole lifecycle of medicines development from the start of the process.’
How integrated guidance helps
All drug development stakeholders including HTA authorities are shaping the future of patient engagement. The FDA, for example, has published a first draft guidance document outlining ways of incorporating the patient voice into their decision-making process. PFMD has advised that the FDA documents would be enhanced by adding complementary guidance on the qualitative perspective of engaging with patients, but broadly welcomed the guide.
Going beyond the HTAi crowd will require revamping the healthcare ecosystem to make it more efficient and more patient centric. There was discussion at the conference on how to make HTA more efficient and effective. However, Brooke says HTA cannot be viewed in isolation: “If our ultimate goal is getting innovations to patients to improve patient outcomes, we cannot think only of improving HTA – we must take a whole-system approach.”
Patient-based evidence
There was also discussion about the link between patient collaboration and patient-based evidence. For Brooke, the two are inextricably linked: you cannot have one without the other.
“Patient collaboration without evidence is not necessarily serving the patients; equally, if you generate patient-based evidence without input from patients you will never know if your data is relevant to patients,” Brooke argued.
He said the HTAi conference showed strong interest in patient-based evidence but that this should not come at the expense of collaboration with patients. “Even with the best methodology in the world and the highest-quality data, if you didn’t ask the right question initially – because you didn’t design it with patients – you cannot get the right results,” he said.
Brooke said patient collaboration and patient-based evidence should be viewed as an “infinite ribbon” with each having to constantly referring back to the other side. It’s an ongoing, never-ending process, he said.
The post-HTA era?
Brooke spoke at the close of a plenary entitled Connecting the Dots, which was captured in a rich and informative sketch. As well as involving patients early and often, it highlights the need to incorporate real-world data. Ultimately, a health system with the capacity to learn using ‘live’ data might not even need HTA at all. Brooke said a self-learning system would still need technology assessment expertise to drive decisions but in any case,‘it is vital that we tackle the whole system, for it is the system that serves the patient’.